My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
32<br />
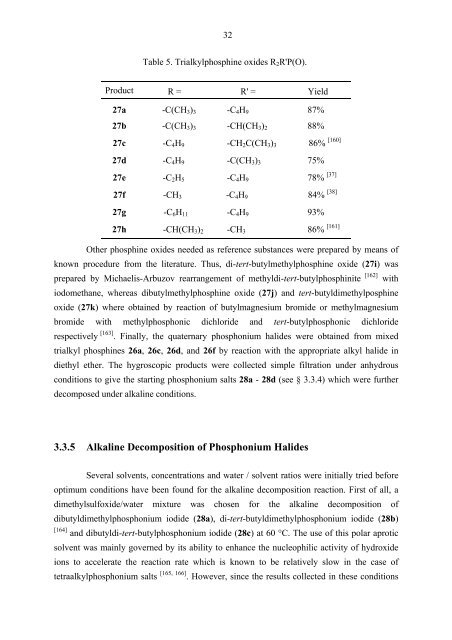
Table 5. Trialkylphosphine oxides R2R'P(O).<br />
Product R = R' = Yield<br />
27a -C(CH3)3 -C4H9 87%<br />
27b -C(CH3)3 -CH(CH3)2 88%<br />
27c -C4H9 -CH2C(CH3)3 86%<br />
27d -C4H9 -C(CH3)3 75%<br />
27e -C2H5 -C4H9 78% [37]<br />
27f -CH3 -C4H9 84% [38]<br />
27g -C6H11 -C4H9 93%<br />
27h -CH(CH3)2 -CH3 86%<br />
Other phosphine oxides needed as reference substances were prepared by means of<br />
known procedure from the literature. Thus, di-tert-butylmethylphosphine oxide (27i) was<br />
prepared by Michaelis-Arbuzov rearrangement of methyldi-tert-butylphosphinite<br />
[ ] 160<br />
[ ] 161<br />
[ ] 162 with<br />
iodomethane, whereas dibutylmethylphosphine oxide (27j) and tert-butyldimethylposphine<br />
oxide (27k) where obtained by reaction of butylmagnesium bromide or methylmagnesium<br />
bromide with methylphosphonic dichloride and tert-butylphosphonic dichloride<br />
respectively<br />
[ ] 163 . Finally, the quaternary phosphonium halides were obtained from mixed<br />
trialkyl phosphines 26a, 26c, 26d, and 26f by reaction with the appropriate alkyl halide in<br />
diethyl ether. The hygroscopic products were collected simple filtration under anhydrous<br />
conditions to give the starting phosphonium salts 28a - 28d (see § 3.3.4) which were further<br />
decomposed under alkaline conditions.<br />
3.3.5 Alkaline Decomposition of Phosphonium Halides<br />
Several solvents, concentrations and water / solvent ratios were initially tried before<br />
optimum conditions have been found for the alkaline decomposition reaction. First of all, a<br />
dimethylsulfoxide/water mixture was chosen for the alkaline decomposition of<br />
dibutyldimethylphosphonium iodide (28a), di-tert-butyldimethylphosphonium iodide (28b)<br />
[ 164 ]<br />
and dibutyldi-tert-butylphosphonium iodide (28c) at 60 °C. The use of this polar aprotic<br />
solvent was mainly governed by its ability to enhance the nucleophilic activity of hydroxide<br />
ions to accelerate the reaction rate which is known to be relatively slow in the case of<br />
tetraalkylphosphonium salts<br />
[ , ] 165 166 . However, since the results collected in these conditions