My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
45<br />
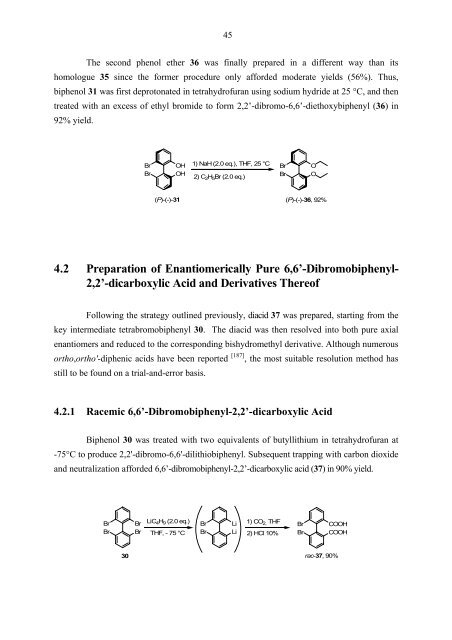
The second phenol ether 36 was finally prepared in a different way than its<br />
homologue 35 since the former procedure only afforded moderate yields (56%). Thus,<br />
biphenol 31 was first deprotonated in tetrahydrofuran using sodium hydride at 25 °C, and then<br />
treated with an excess of ethyl bromide to form 2,2’-dibromo-6,6’-diethoxybiphenyl (36) in<br />
92% yield.<br />
Br<br />
Br<br />
OH<br />
OH<br />
1) NaH (2. 0 eq.), THF, 25 °C<br />
2) C2H 5Br<br />
(2.0 eq.)<br />
Br<br />
Br<br />
O<br />
O<br />
(P)-(-)-31 (P)-(-)-36, 92%<br />
4.2 Preparation of Enantiomerically Pure 6,6’-Dibromobiphenyl-<br />
2,2’-dicarboxylic Acid and Derivatives Thereof<br />
Following the strategy outlined previously, diacid 37 was prepared, starting from the<br />
key intermediate tetrabromobiphenyl 30. The diacid was then resolved into both pure axial<br />
enantiomers and reduced to the corresponding bishydromethyl derivative. Although numerous<br />
ortho,ortho'-diphenic acids have been reported<br />
still to be found on a trial-and-error basis.<br />
[ ] 187 , the most suitable resolution method has<br />
4.2.1 Racemic 6,6’-Dibromobiphenyl-2,2’-dicarboxylic Acid<br />
Biphenol 30 was treated with two equivalents of butyllithium in tetrahydrofuran at<br />
-75°C to produce 2,2'-dibromo-6,6'-dilithiobiphenyl. Subsequent trapping with carbon dioxide<br />
and neutralization afforded 6,6’-dibromobiphenyl-2,2’-dicarboxylic acid (37) in 90% yield.<br />
Br Br<br />
Br Br<br />
30<br />
LiC 4H 9 (2.0 eq.)<br />
THF, - 75 °C<br />
Br Li<br />
Br Li<br />
1) CO 2, THF<br />
2) HCl 10%<br />
Br COOH<br />
Br COOH<br />
rac-37, 90%