My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
48<br />
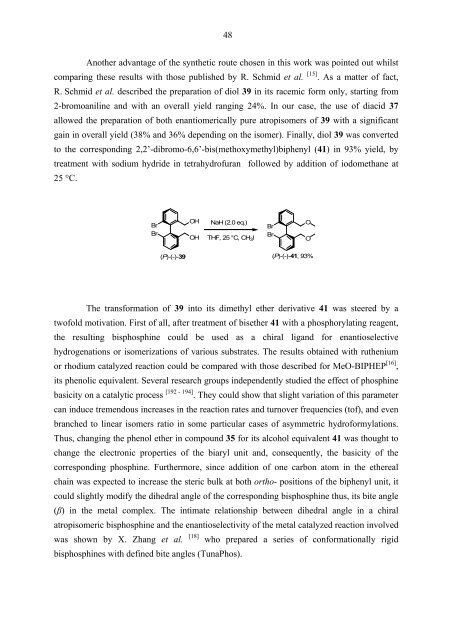
Another advantage of the synthetic route chosen in this work was pointed out whilst<br />
comparing these results with those published by R. Schmid et al. [15] . As a matter of fact,<br />
R. Schmid et al. described the preparation of diol 39 in its racemic form only, starting from<br />
2-bromoaniline and with an overall yield ranging 24%. In our case, the use of diacid 37<br />
allowed the preparation of both enantiomerically pure atropisomers of 39 with a significant<br />
gain in overall yield (38% and 36% depending on the isomer). Finally, diol 39 was converted<br />
to the corresponding 2,2’-dibromo-6,6’-bis(methoxymethyl)biphenyl (41) in 93% yield, by<br />
treatment with sodium hydride in tetrahydrofuran followed by addition of iodomethane at<br />
25 °C.<br />
Br<br />
Br<br />
(P)-(-)-39<br />
OH<br />
OH<br />
NaH (2.0 eq.)<br />
THF, 25 °C, CH 3I<br />
Br<br />
Br<br />
O<br />
O<br />
(P)-(-)-41, 93%<br />
The transformation of 39 into its dimethyl ether derivative 41 was steered by a<br />
twofold motivation. First of all, after treatment of bisether 41 with a phosphorylating reagent,<br />
the resulting bisphosphine could be used as a chiral ligand for enantioselective<br />
hydrogenations or isomerizations of various substrates. The results obtained with ruthenium<br />
or rhodium catalyzed reaction could be compared with those described for MeO-BIPHEP [16] ,<br />
its phenolic equivalent. Several research groups independently studied the effect of phosphine<br />
basicity on a catalytic process<br />
[ - ] 192 194 . They could show that slight variation of this parameter<br />
can induce tremendous increases in the reaction rates and turnover frequencies (tof), and even<br />
branched to linear isomers ratio in some particular cases of asymmetric hydroformylations.<br />
Thus, changing the phenol ether in compound 35 for its alcohol equivalent 41 was thought to<br />
change the electronic properties of the biaryl unit and, consequently, the basicity of the<br />
corresponding phosphine. Furthermore, since addition of one carbon atom in the ethereal<br />
chain was expected to increase the steric bulk at both ortho- positions of the biphenyl unit, it<br />
could slightly modify the dihedral angle of the corresponding bisphosphine thus, its bite angle<br />
(β) in the metal complex. The intimate relationship between dihedral angle in a chiral<br />
atropisomeric bisphosphine and the enantioselectivity of the metal catalyzed reaction involved<br />
was shown by X. Zhang et al. [18] who prepared a series of conformationally rigid<br />
bisphosphines with defined bite angles (TunaPhos).