My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
26<br />
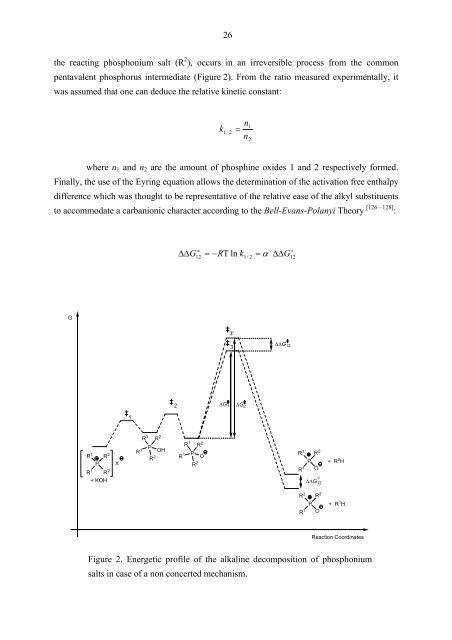
the reacting phosphonium salt (R 2 ), occurs in an irreversible process from the common<br />
pentavalent phosphorus intermediate (Figure 2). From the ratio measured experimentally, it<br />
was assumed that one can deduce the relative kinetic constant:<br />
n<br />
k 1/<br />
2 =<br />
n<br />
where n1 and n2 are the amount of phosphine oxides 1 and 2 respectively formed.<br />
Finally, the use of the Eyring equation allows the determination of the activation free enthalpy<br />
difference which was thought to be representative of the relative ease of the alkyl substituents<br />
to accommodate a carbanionic character according to the Bell-Evans-Polanyi Theory [126 - 128] :<br />
G<br />
R 1<br />
P<br />
R 2<br />
R 1 R 2<br />
+ KOH<br />
X<br />
1<br />
R 1<br />
R 1<br />
R 2<br />
P<br />
R2 OH<br />
2<br />
∆∆ 12<br />
≠<br />
R 1<br />
R 1<br />
G = −RT<br />
ln k = α ∆∆G<br />
R 2<br />
P<br />
R2 O<br />
∆G 1<br />
3'<br />
3<br />
∆G 2<br />
1<br />
2<br />
1/<br />
2<br />
∆∆G 12<br />
o<br />
12<br />
R 1 R 2<br />
P<br />
R O 1<br />
0<br />
∆∆G12 R<br />
P<br />
O<br />
2 R 2<br />
R1 + R 2 H<br />
+ R 1 H<br />
Reaction Coordinates<br />
Figure 2. Energetic profile of the alkaline decomposition of phosphonium<br />
salts in case of a non concerted mechanism.