My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
3.3.1 Mechanistic Discussion on the Alkaline Decomposition of<br />
Phosphonium Salts<br />
23<br />
Although various authors [29 - 35] assumed this decomposition to proceed trough<br />
several independent equilibrated or irreversible steps, the kinetics and other results reported<br />
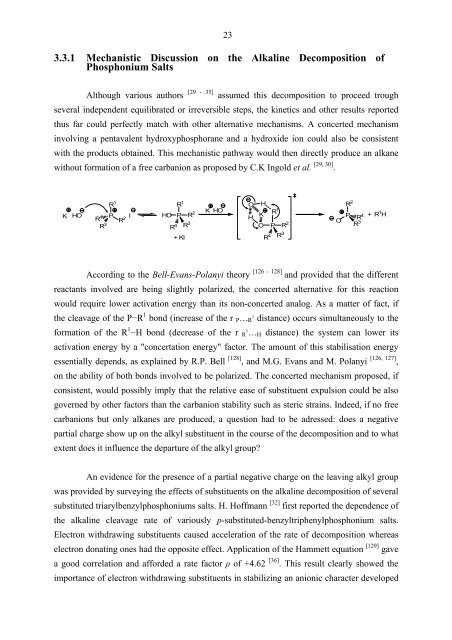
thus far could perfectly match with other alternative mechanisms. A concerted mechanism<br />
involving a pentavalent hydroxyphosphorane and a hydroxide ion could also be consistent<br />
with the products obtained. This mechanistic pathway would then directly produce an alkane<br />
without formation of a free carbanion as proposed by C.K Ingold et al. [29, 30] .<br />
R 1<br />
P<br />
R3 R 4<br />
R2 K HO<br />
I<br />
HO<br />
R 4<br />
R 1<br />
P<br />
+ KI<br />
R 3<br />
R 2<br />
K HO<br />
R1 R2 O H<br />
H<br />
K<br />
O P<br />
According to the Bell-Evans-Polanyi theory<br />
R 4<br />
R 3<br />
R 2<br />
P<br />
R3R4 + R1H O<br />
[ - ] 126 128 and provided that the different<br />
reactants involved are being slightly polarized, the concerted alternative for this reaction<br />
would require lower activation energy than its non-concerted analog. As a matter of fact, if<br />
the cleavage of the P−R 1 bond (increase of the r P…R 1 distance) occurs simultaneously to the<br />
formation of the R 1 −H bond (decrease of the r R 1 …H distance) the system can lower its<br />
activation energy by a "concertation energy" factor. The amount of this stabilisation energy<br />
essentially depends, as explained by R.P. Bell [128] , and M.G. Evans and M. Polanyi [126, 127] ,<br />
on the ability of both bonds involved to be polarized. The concerted mechanism proposed, if<br />
consistent, would possibly imply that the relative ease of substituent expulsion could be also<br />
governed by other factors than the carbanion stability such as steric strains. Indeed, if no free<br />
carbanions but only alkanes are produced, a question had to be adressed: does a negative<br />
partial charge show up on the alkyl substituent in the course of the decomposition and to what<br />
extent does it influence the departure of the alkyl group?<br />
An evidence for the presence of a partial negative charge on the leaving alkyl group<br />
was provided by surveying the effects of substituents on the alkaline decomposition of several<br />
substituted triarylbenzylphosphoniums salts. H. Hoffmann [32] first reported the dependence of<br />
the alkaline cleavage rate of variously p-substituted-benzyltriphenylphosphonium salts.<br />
Electron withdrawing substituents caused acceleration of the rate of decomposition whereas<br />
electron donating ones had the opposite effect. Application of the Hammett equation<br />
[ ] 129 gave<br />
a good correlation and afforded a rate factor ρ of +4.62 [36] . This result clearly showed the<br />
importance of electron withdrawing substituents in stabilizing an anionic character developed