My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
44<br />
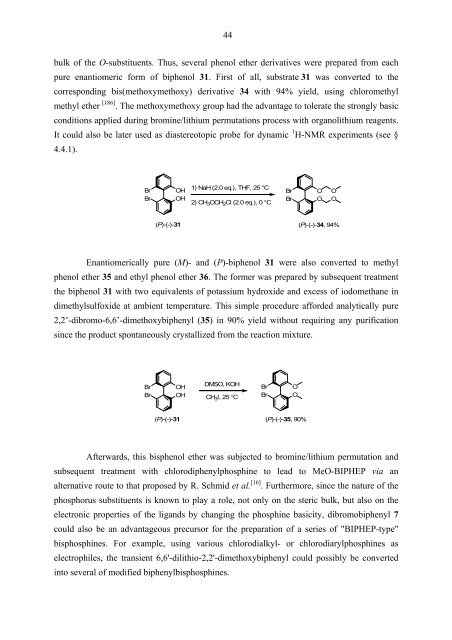
bulk of the O-substituents. Thus, several phenol ether derivatives were prepared from each<br />
pure enantiomeric form of biphenol 31. First of all, substrate 31 was converted to the<br />
corresponding bis(methoxymethoxy) derivative 34 with 94% yield, using chloromethyl<br />
methyl ether<br />
[ ] 186 . The methoxymethoxy group had the advantage to tolerate the strongly basic<br />
conditions applied during bromine/lithium permutations process with organolithium reagents.<br />
It could also be later used as diastereotopic probe for dynamic 1 H-NMR experiments (see §<br />
4.4.1).<br />
Br<br />
Br<br />
OH<br />
OH<br />
(P)-(-)-31<br />
1) NaH (2.0 eq.), THF, 25 °C<br />
2) CH 3OCH<br />
2Cl (2.0 eq.), 0 °C<br />
Br O O<br />
Br O O<br />
(P)-(-)-34, 94%<br />
Enantiomerically pure (M)- and (P)-biphenol 31 were also converted to methyl<br />
phenol ether 35 and ethyl phenol ether 36. The former was prepared by subsequent treatment<br />
the biphenol 31 with two equivalents of potassium hydroxide and excess of iodomethane in<br />
dimethylsulfoxide at ambient temperature. This simple procedure afforded analytically pure<br />
2,2’-dibromo-6,6’-dimethoxybiphenyl (35) in 90% yield without requiring any purification<br />
since the product spontaneously crystallized from the reaction mixture.<br />
Br<br />
Br<br />
OH<br />
OH<br />
DMSO, KOH<br />
CH3I, 25 °C<br />
Br<br />
Br<br />
O<br />
O<br />
(P)-(-)-31 (P)-(-)-35, 90%<br />
Afterwards, this bisphenol ether was subjected to bromine/lithium permutation and<br />
subsequent treatment with chlorodiphenylphosphine to lead to MeO-BIPHEP via an<br />
alternative route to that proposed by R. Schmid et al. [16] . Furthermore, since the nature of the<br />
phosphorus substituents is known to play a role, not only on the steric bulk, but also on the<br />
electronic properties of the ligands by changing the phosphine basicity, dibromobiphenyl 7<br />
could also be an advantageous precursor for the preparation of a series of "BIPHEP-type"<br />
bisphosphines. For example, using various chlorodialkyl- or chlorodiarylphosphines as<br />
electrophiles, the transient 6,6'-dilithio-2,2'-dimethoxybiphenyl could possibly be converted<br />
into several of modified biphenylbisphosphines.