My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
7<br />
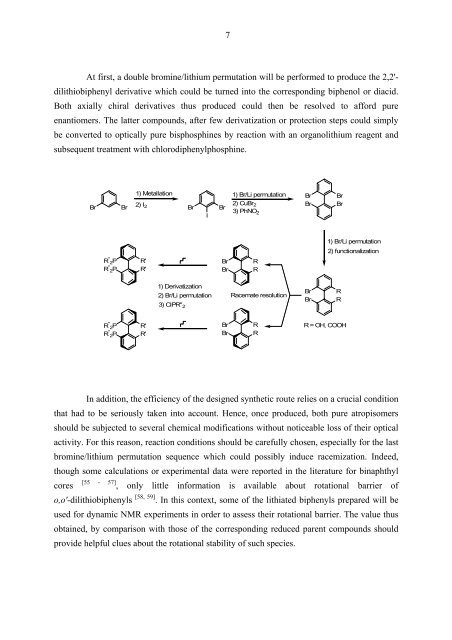
At first, a double bromine/lithium permutation will be performed to produce the 2,2'-<br />
dilithiobiphenyl derivative which could be turned into the corresponding biphenol or diacid.<br />
Both axially chiral derivatives thus produced could then be resolved to afford pure<br />
enantiomers. The latter compounds, after few derivatization or protection steps could simply<br />
be converted to optically pure bisphosphines by reaction with an organolithium reagent and<br />
subsequent treatment with chlorodiphenylphosphine.<br />
R'' 2P<br />
R'' 2P<br />
R'<br />
R'<br />
R R'<br />
'' 2P<br />
R'' 2P R'<br />
1) Metallation<br />
Br Br<br />
2) I2 Br Br<br />
I<br />
1) Derivatization<br />
2) Br/Li permutation<br />
3) ClPR'' 2<br />
Br<br />
Br<br />
1) Br/Li permutation<br />
2) CuBr 2<br />
3) PhNO 2<br />
R<br />
R<br />
Racemate resolution<br />
Br R<br />
Br R<br />
Br Br<br />
Br Br<br />
Br R<br />
Br R<br />
R = OH, COOH<br />
1) Br/Li permutation<br />
2) functionalization<br />
In addition, the efficiency of the designed synthetic route relies on a crucial condition<br />
that had to be seriously taken into account. Hence, once produced, both pure atropisomers<br />
should be subjected to several chemical modifications without noticeable loss of their optical<br />
activity. For this reason, reaction conditions should be carefully chosen, especially for the last<br />
bromine/lithium permutation sequence which could possibly induce racemization. Indeed,<br />
though some calculations or experimental data were reported in the literature for binaphthyl<br />
cores<br />
[ 55 - 57 ]<br />
, only little information is available about rotational barrier of<br />
o,o'-dilithiobiphenyls<br />
[ , ] 58 59 . In this context, some of the lithiated biphenyls prepared will be<br />
used for dynamic NMR experiments in order to assess their rotational barrier. The value thus<br />
obtained, by comparison with those of the corresponding reduced parent compounds should<br />
provide helpful clues about the rotational stability of such species.