My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
My PhD dissertation - Institut Fresnel
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
29<br />
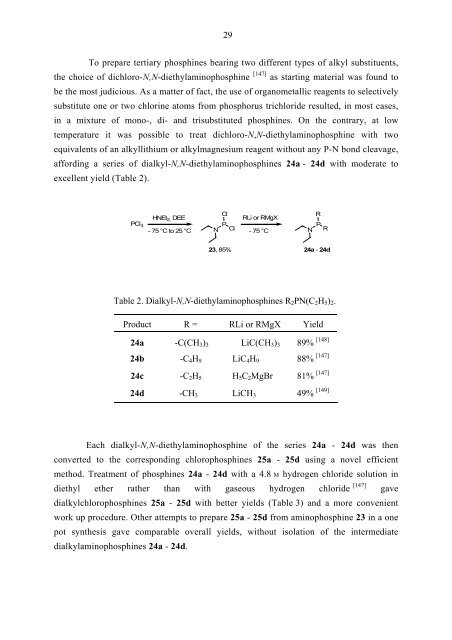
To prepare tertiary phosphines bearing two different types of alkyl substituents,<br />
the choice of dichloro-N,N-diethylaminophosphine<br />
[ ] 147 as starting material was found to<br />
be the most judicious. As a matter of fact, the use of organometallic reagents to selectively<br />
substitute one or two chlorine atoms from phosphorus trichloride resulted, in most cases,<br />
in a mixture of mono-, di- and trisubstituted phosphines. On the contrary, at low<br />
temperature it was possible to treat dichloro-N,N-diethylaminophosphine with two<br />
equivalents of an alkyllithium or alkylmagnesium reagent without any P-N bond cleavage,<br />
affording a series of dialkyl-N,N-diethylaminophosphines 24a - 24d with moderate to<br />
excellent yield (Table 2).<br />
PCl 3<br />
HNEt 2, DEE<br />
- 75 °C to 25 °C<br />
Cl<br />
P<br />
N Cl<br />
23, 85%<br />
RLi or RMgX<br />
- 75 °C<br />
R<br />
P<br />
N R<br />
24a - 24d<br />
Table 2. Dialkyl-N,N-diethylaminophosphines R2PN(C2H5)2.<br />
Product R = RLi or RMgX Yield<br />
24a -C(CH3)3 LiC(CH3)3 89%<br />
[ ] 148<br />
24b -C4H9 LiC4H9 88% [147]<br />
24c -C2H5 H5C2MgBr 81% [147]<br />
24d -CH3 LiCH3 49%<br />
Each dialkyl-N,N-diethylaminophosphine of the series 24a - 24d was then<br />
converted to the corresponding chlorophosphines 25a - 25d using a novel efficient<br />
method. Treatment of phosphines 24a - 24d with a 4.8 M hydrogen chloride solution in<br />
diethyl ether rather than with gaseous hydrogen chloride [147] gave<br />
dialkylchlorophosphines 25a - 25d with better yields (Table 3) and a more convenient<br />
work up procedure. Other attempts to prepare 25a - 25d from aminophosphine 23 in a one<br />
pot synthesis gave comparable overall yields, without isolation of the intermediate<br />
dialkylaminophosphines 24a - 24d.<br />
[ ] 149