Geologic Studies in Alaska by the U.S. Geological Survey, 1992
Geologic Studies in Alaska by the U.S. Geological Survey, 1992
Geologic Studies in Alaska by the U.S. Geological Survey, 1992
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
192<br />
GEOLOGIC STUDIES IN ALASKA BY THE U.S. GEOLOGICAL SURVEY, <strong>1992</strong><br />
estimated several percent of iron <strong>in</strong>dicated for this phase<br />
would be most unusual for albite, especially a presumed<br />
low-temperature albite, <strong>in</strong>asmuch as pegmatitic albites<br />
typically have less than 0.01 percent iron (Smith, 1983).<br />
Possibly <strong>the</strong> phase is a quenched iron-bear<strong>in</strong>g glass, but<br />
<strong>the</strong>n its lack of potassium might seem puzzl<strong>in</strong>g.<br />
In fact, a potassic m<strong>in</strong>eral is present on <strong>the</strong> smooth<br />
areas on <strong>the</strong> natural fracture surface of CM rock sample<br />
92DB-05C where it occurs as (light gray) hexagonal plates<br />
(fig. 9A). It is even more apparent <strong>in</strong> <strong>the</strong> gray area at <strong>the</strong><br />
lower left of figure 9A. The area, shown enlarged <strong>in</strong> <strong>the</strong><br />
upper right of figure 9B, can be seen to consist of two<br />
phases. The darker areas show<strong>in</strong>g between <strong>the</strong> hexagons<br />
<strong>in</strong> figure 9B are of (iron-free) albite composition (ab). The<br />
lighter phase (kim) conta<strong>in</strong>s abundant potassium (fig. 11).<br />
Over a range of a factor of five <strong>in</strong> potassium peak <strong>in</strong>ten-<br />
sity, FeK is acceptably <strong>in</strong>variant (3 to 3.6) for 9 of 12<br />
analyses. Similarly, eight analyses gave MoK peak <strong>in</strong>ten-<br />
sities from 2.1 to 3.1. In contrast, SiIK <strong>in</strong>tensities ranged<br />
from 1 to 27 and Si/Al <strong>in</strong>tensities are close to those for<br />
albite. We conclude that <strong>the</strong> spectrum shown <strong>in</strong> figure 11<br />
likely represents a mixture of albite and a non-silicate m<strong>in</strong>-<br />
eral conta<strong>in</strong><strong>in</strong>g potassium, iron, and molybdenum. Be-<br />
cause <strong>the</strong> readily observed X-ray peaks for molybdenum<br />
and sulfur have overlapp<strong>in</strong>g energies that cannot be easily<br />
dist<strong>in</strong>guished, <strong>the</strong> m<strong>in</strong>eral could be a sulfide. It also could<br />
be an oxide or hydroxide among o<strong>the</strong>r possibilities.<br />
Ferrimolybdite is a known wea<strong>the</strong>r<strong>in</strong>g product, and <strong>the</strong>re<br />
are several potassic molybdates. This raises <strong>the</strong> question<br />
of <strong>the</strong> orig<strong>in</strong> of thls hexagonal phase. Because we are ana-<br />
lyz<strong>in</strong>g a natural, unpolished surface, <strong>the</strong> phase almost cer-<br />
ta<strong>in</strong>ly represents some k<strong>in</strong>d of surficial deposit or reaction.<br />
The hexagons appear to be oriented parallel to <strong>the</strong> surface<br />
and nucleation appears to have dom<strong>in</strong>ated crystal growth<br />
k<strong>in</strong>etics (that is, <strong>the</strong>re are numerous small crystals). If<br />
<strong>the</strong>y are wea<strong>the</strong>r<strong>in</strong>g products, a source of potassium would<br />
be required, and this could be <strong>the</strong> potassium feldspar con-<br />
ta<strong>in</strong>ed <strong>in</strong> <strong>the</strong> bulk rock. Ano<strong>the</strong>r possibility is that this<br />
potassium-iron-molybdenum phase may have crystallized<br />
(perhaps subsolidus) as part of a system quenched <strong>in</strong> a<br />
manner similar to that ascribed above to <strong>the</strong> conjectured<br />
iron-albite glass.<br />
DISCUSSION<br />
The Dora Bay molybdenite occurrences are only<br />
show<strong>in</strong>gs, but molybdenite occurs here <strong>in</strong> similar sett<strong>in</strong>gs<br />
at two localities that are 3 km apart; readily visible molyb-<br />
denite occurs over a width of about 5 m <strong>in</strong> <strong>the</strong> HM pit<br />
face; and analyses of two bulk HM samples yielded 0.5<br />
and 1.2 percent molybdenum, respectively. These discov-<br />
eries offer at least some prospect of a molybdenite deposit<br />
<strong>in</strong> <strong>the</strong> vic<strong>in</strong>ity. At our current level of understand<strong>in</strong>g, <strong>the</strong><br />
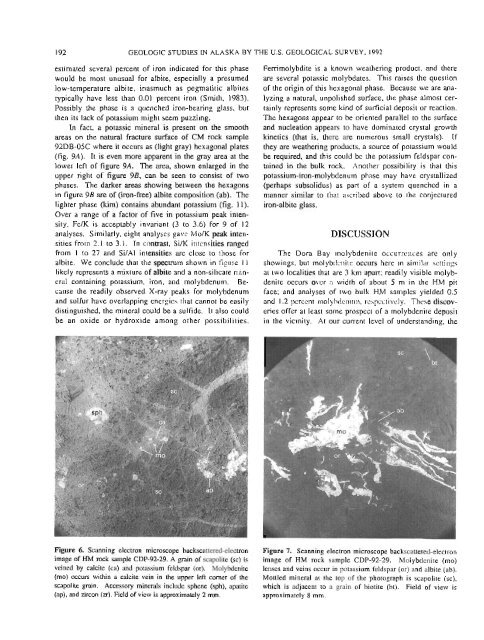
Figure 6. Scann<strong>in</strong>g electron microscope backscattered-electron Figure 7. Scann<strong>in</strong>g electron microscope backscattered-electron<br />
image of HM rock sample CDP-92-29. A gra<strong>in</strong> of scapolite (sc) is image of HM rock sample CDP-92-29. Molybdenite (mo)<br />
ve<strong>in</strong>ed <strong>by</strong> calcite (ca) and potassium feldspar (or). Molybdenite lenses and ve<strong>in</strong>s occur <strong>in</strong> potassium feldspar (or) and albite (ab).<br />
(mo) occurs with<strong>in</strong> a calcite ve<strong>in</strong> <strong>in</strong> <strong>the</strong> upper left comer of <strong>the</strong> Mottled m<strong>in</strong>eral at <strong>the</strong> top of <strong>the</strong> photograph is scapolite (sc),<br />
scapolite gra<strong>in</strong>. Accessory m<strong>in</strong>erals <strong>in</strong>cl~ide sphene (sph), apatite which is adjacent to a gra<strong>in</strong> of biotite (bt). Field of view is<br />
(ap), and zircon (zr). Field of view is approximately 2 mm. approximately 8 mm.