Geologic Studies in Alaska by the U.S. Geological Survey, 1992
Geologic Studies in Alaska by the U.S. Geological Survey, 1992
Geologic Studies in Alaska by the U.S. Geological Survey, 1992
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
238 GEOLOGIC STUDIES IN ALASKA BY THE U.S. GEOLOGICAL SURVEY, <strong>1992</strong><br />
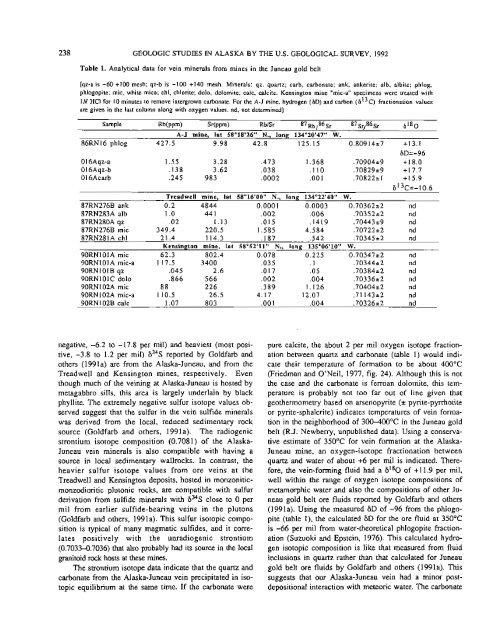
Table 1. Analytical data for ve<strong>in</strong> m<strong>in</strong>erals from m<strong>in</strong>es <strong>in</strong> <strong>the</strong> Juneau gold belt<br />
[qz-a is -60 +I00 mesh; qz-b is -100 +I40 mesh. M<strong>in</strong>erals: qz, quartz; carb, carbonate; ank, ankerite; alb, albite; phlog,<br />
phlogopite; mic, white mica; chl, chlorite; dolo, dolomite; calc, calcite. Kens<strong>in</strong>gton m<strong>in</strong>e "mic-a" specimens were treated with<br />
1N HCI for 10 m<strong>in</strong>utes to remove <strong>in</strong>tergrown carbonate. For <strong>the</strong> A-J m<strong>in</strong>e, hydrogen (GD) and carbon (G13c) fractionation values<br />
are given <strong>in</strong> <strong>the</strong> last column along with oxygen values. nd, not determ<strong>in</strong>ed]<br />
Sample Rb(PPm) Sr(PPm) RbISr 87~h - -- ,86s,<br />
I --<br />
87~,,86~,<br />
--I --<br />
~180 - -<br />
A-J m<strong>in</strong>e, lat S8°18'26" N., long 134"20'47" W.<br />
86RN16 phlog 427.5 9.98 42.8 125.15 0.80914*7 +13.1<br />
6D=-96<br />
01 6Aqz-a 1.55 3.28 .473 1.368 .7090429 +18.0<br />
01 6Aqz-b<br />
0 1 6Acarb<br />
.I38<br />
.245<br />
3.62<br />
983<br />
.038<br />
.0002<br />
.I10<br />
.001<br />
.70829*9<br />
.70822*1<br />
+17.7<br />
+15.9<br />
613c=-10.6<br />
Treadwell m<strong>in</strong>e, lat 58"16'0OW N., long 134"22'40" W.<br />
87RN276B ank 0.2 4844 0.0001 0.0003 0.7036222 nd<br />
87RN283A alb 1 .O 44 1 .002 .006 .70352+.2 nd<br />
87RN280A qz .02 1.13 .O 15 .I419 .70443*9 nd<br />
87RN276B mic 349.4 220.5 1.585 4.584 ,7072222 nd<br />
87RN281A chl 21.4 114.3 .I87 .542 .70345*2 nd<br />
Kens<strong>in</strong>gton m<strong>in</strong>e, lat 58°S2'11" N., long 13S006'10" W.<br />
90RN101A mic 62.3 802.4 0.078 0.225 0.7034722 nd<br />
90RN101A mic-a 117.5 3400 ,035 .1 .70344+2 nd<br />
90RN101B qz .045 2.6 .017 .05 .70384+2 nd<br />
90RN 10 1 C dolo .866 566 .002 .004 .70336+2 nd<br />
90RN102A mic 8 8 226 .389 1.126 .70404+2 nd<br />
90RN102A mic-a 110.5 26.5 4.17 12.07 .71143+2 nd<br />
90RN 102B calc 1.07 803 .OO 1 ,004 .70326r2 nd<br />
negative, -6.2 to -17.8 per mil) and heaviest (most positive,<br />
-3.8 to 1.2 per mil) S34~ reported <strong>by</strong> Goldfarb and<br />
o<strong>the</strong>rs (1991a) are from <strong>the</strong> <strong>Alaska</strong>-Juneau, and from <strong>the</strong><br />
Treadwell and Kens<strong>in</strong>gton m<strong>in</strong>es, respectively. Even<br />
though much of <strong>the</strong> ve<strong>in</strong><strong>in</strong>g at <strong>Alaska</strong>-Juneau is hosted <strong>by</strong><br />
metagabbro sills, this area is largely underla<strong>in</strong> <strong>by</strong> black<br />
phyllite. The extremely negative sulfur isotope values observed<br />
suggest that <strong>the</strong> sulfur <strong>in</strong> <strong>the</strong> ve<strong>in</strong> sulfide m<strong>in</strong>erals<br />
was derived from <strong>the</strong> local, reduced sedimentary rock<br />
source (Goldfarb and o<strong>the</strong>rs, 1991a). The radiogenic<br />
strontium isotope composition (0.7081) of <strong>the</strong> <strong>Alaska</strong>-<br />
Juneau ve<strong>in</strong> m<strong>in</strong>erals is also compatible with hav<strong>in</strong>g a<br />
source <strong>in</strong> local sedimentary wallrocks. In contrast, <strong>the</strong><br />
heavier sulfur isotope values from ore ve<strong>in</strong>s at <strong>the</strong><br />
Treadwell and Kens<strong>in</strong>gton deposits, hosted <strong>in</strong> monzoniticmonzodioritic<br />
plutonic rocks, are compatible with sulfur<br />
derivation from sulfide m<strong>in</strong>erals with 634~ close to 0 per<br />
mil from earlier sulfide-bear<strong>in</strong>g ve<strong>in</strong>s <strong>in</strong> <strong>the</strong> plutons<br />
(Goldfarb and o<strong>the</strong>rs, 1991a). This sulfur isotopic composition<br />
is typical of many magmatic sulfides, and it correlates<br />
positively with <strong>the</strong> unradiogenic strontium<br />
(0.7033-0.7036) that also probably had its source <strong>in</strong> <strong>the</strong> local<br />
granitoid rock hosts at <strong>the</strong>se m<strong>in</strong>es.<br />
The strontium isotope data <strong>in</strong>dicate that <strong>the</strong> quartz and<br />
carbonate from <strong>the</strong> <strong>Alaska</strong>-Juneau ve<strong>in</strong> precipitated <strong>in</strong> isotopic<br />
equilibrium at <strong>the</strong> same time. If <strong>the</strong> carbonate were<br />
pure calcite, <strong>the</strong> about 2 per mil oxygen isotope fraction-<br />
ation between quartz and carbonate (table 1) would <strong>in</strong>di-<br />
cate <strong>the</strong>ir temperature of formation to be about 400°C<br />
(Friedman and O'Neil, 1977, fig. 24). Although this is not<br />
<strong>the</strong> case and <strong>the</strong> carbonate is ferroan dolomite, this tem-<br />
perature is probably not too far out of l<strong>in</strong>e given that<br />
geo<strong>the</strong>rmometry based on arsenopyrite (k pyrite-pyrrhotite<br />
or pyrite-sphalerite) <strong>in</strong>dicates temperatures of ve<strong>in</strong> forma-<br />
tion <strong>in</strong> <strong>the</strong> neighborhood of 300400°C <strong>in</strong> <strong>the</strong> Juneau gold<br />
belt (R.J. Newberry, unpublished data). Us<strong>in</strong>g a conserva-<br />
tive estimate of 350°C for ve<strong>in</strong> formation at <strong>the</strong> <strong>Alaska</strong>-<br />
Juneau m<strong>in</strong>e, an oxygen-isotope fractionation between<br />
quartz and water of about +6 per mil is <strong>in</strong>dicated. There-<br />
fore, <strong>the</strong> ve<strong>in</strong>-form<strong>in</strong>g fluid had a 6180 of +11.9 per mil,<br />
well with<strong>in</strong> <strong>the</strong> range of oxygen isotope compositions of<br />
metamorphic water and also <strong>the</strong> compositions of o<strong>the</strong>r Ju-<br />
neau gold belt ore fluids reported <strong>by</strong> Goldfarb and o<strong>the</strong>rs<br />
(1991a). Us<strong>in</strong>g <strong>the</strong> measured SD of -96 from <strong>the</strong> phlogo-<br />
pite (table I), <strong>the</strong> calculated 6D for <strong>the</strong> ore fluid at 350°C<br />
is -66 per mil from water-<strong>the</strong>oretical phlogopite fraction-<br />
ation (Suzuoki and Epste<strong>in</strong>, 1976). This calculated hydro-<br />
gen isotopic composition is like that measured from fluid<br />
<strong>in</strong>clusions <strong>in</strong> quartz ra<strong>the</strong>r than that calculated for Juneau<br />
gold belt ore fluids <strong>by</strong> Goldfarb and o<strong>the</strong>rs (1991a). This<br />
suggests that our <strong>Alaska</strong>-Juneau ve<strong>in</strong> had a m<strong>in</strong>or post-<br />
depositional <strong>in</strong>teraction with meteoric water. The carbonate