Eble JN, Sauter G., Epstein JI, Sesterhenn IA - iarc
Eble JN, Sauter G., Epstein JI, Sesterhenn IA - iarc
Eble JN, Sauter G., Epstein JI, Sesterhenn IA - iarc
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Similar tumours as those of group 1 and<br />
2 can be found in the ovary and extragonadal<br />
sites, in particular along the midline<br />
of the body. Relatively little is known<br />
on the genomic changes of these GCTs.<br />
Supposedly the findings in the GCTs of<br />
the testis are also relevant for classification<br />
and understanding of the pathogenesis<br />
of ovarian and extragonadal GCTs.<br />
Genetic susceptibility (familial tumours)<br />
Familial testicular germ cell tumours of<br />
adolescents and adults (TGCTs), account<br />
for 1.5-2% of all germ cell tumours of<br />
adults. The familial risks of TGCTs<br />
increase 3.8-fold for fathers, 8.3 for brothers<br />
and 3.9 for sons indicating that genetic<br />
predisposition is a contributor to testicular<br />
cancer {532}. Earlier age of onset, a<br />
higher frequency of bilaterality and an<br />
increased severity of disease suggest<br />
that genetic anticipation is responsible<br />
for many father-son TGCTs {1014}.<br />
Recently, environmental and heritable<br />
causes of cancer have been analysed by<br />
structural equation modelling {532}. The<br />
estimate of proportion of cancer susceptibility<br />
due to genetic effects was 25% in<br />
adult TGCTs. The childhood shared environmental<br />
effects were also important in<br />
testicular cancer (17%).<br />
Numerous groups have attempted to<br />
identify candidate regions for a TGCT<br />
susceptibility gene or genes {1386,1457,<br />
2148,2435}. No differences were detected<br />
between familial/bilateral and sporadic<br />
TGCT in chromosomal changes {2435}.<br />
However, a TGCT susceptibility gene on<br />
chromosome Xq27, that also predisposes<br />
to undescended testis, has been proposed<br />
by the International Testicular<br />
Cancer Linkage Consortium {2148}.<br />
Although the role of genetic factors in the<br />
etiology of TGCTs appears to be established,<br />
the existence of a single susceptibility<br />
gene is doubtful. Most probably<br />
genetic predisposition shared with<br />
intrauterine or childhood environmental<br />
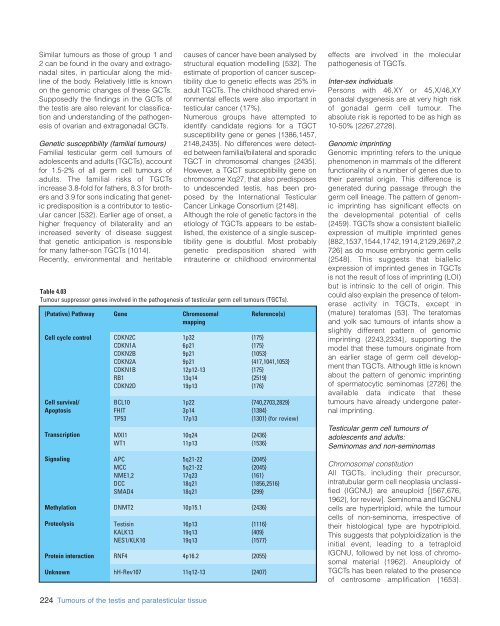
Table 4.03<br />
Tumour suppressor genes involved in the pathogenesis of testicular germ cell tumours (TGCTs).<br />
(Putative) Pathway Gene Chromosomal<br />
mapping<br />
Cell cycle control<br />
Cell survival/<br />
Apoptosis<br />
Transcription<br />
Signaling<br />
Methylation<br />
Proteolysis<br />
Protein interaction<br />
Unknown<br />
CDKN2C<br />
CDKN1A<br />
CDKN2B<br />
CDKN2A<br />
CDKN1B<br />
RB1<br />
CDKN2D<br />
BCL10<br />
FHIT<br />
TP53<br />
MXI1<br />
WT1<br />
APC<br />
MCC<br />
NME1,2<br />
DCC<br />
SMAD4<br />
1p32<br />
6p21<br />
9p21<br />
9p21<br />
12p12-13<br />
13q14<br />
19p13<br />
1p22<br />
3p14<br />
17p13<br />
10q24<br />
11p13<br />
5q21-22<br />
5q21-22<br />
17q23<br />
18q21<br />
18q21<br />
RNF4 4p16.2 {2055}<br />
hH-Rev107 11q12-13 {2407}<br />
{175}<br />
{175}<br />
{1053}<br />
{417,1041,1053}<br />
{175}<br />
{2519}<br />
{176}<br />
{740,2703,2829}<br />
{1384}<br />
{1301} (for review)<br />
{2436}<br />
{1536}<br />
{2045}<br />
{2045}<br />
{161}<br />
{1856,2516}<br />
{299}<br />
DNMT2 10p15.1 {2436}<br />
Testisin<br />
KALK13<br />
NES1/KLK10<br />
16p13<br />
19q13<br />
19q13<br />
Reference(s)<br />
{1116}<br />
{409}<br />
{1577}<br />
effects are involved in the molecular<br />
pathogenesis of TGCTs.<br />
Inter-sex individuals<br />
Persons with 46,XY or 45,X/46,XY<br />
gonadal dysgenesis are at very high risk<br />
of gonadal germ cell tumour. The<br />
absolute risk is reported to be as high as<br />
10-50% {2267,2728}.<br />
Genomic imprinting<br />
Genomic imprinting refers to the unique<br />
phenomenon in mammals of the different<br />
functionality of a number of genes due to<br />
their parental origin. This difference is<br />
generated during passage through the<br />
germ cell lineage. The pattern of genomic<br />
imprinting has significant effects on<br />
the developmental potential of cells<br />
{2459}. TGCTs show a consistent biallelic<br />
expression of multiple imprinted genes<br />
{882,1537,1544,1742,1914,2129,2697,2<br />
726} as do mouse embryonic germ cells<br />
{2548}. This suggests that biallelic<br />
expression of imprinted genes in TGCTs<br />
is not the result of loss of imprinting (LOI)<br />
but is intrinsic to the cell of origin. This<br />
could also explain the presence of telomerase<br />
activity in TGCTs, except in<br />
(mature) teratomas {53}. The teratomas<br />
and yolk sac tumours of infants show a<br />
slightly different pattern of genomic<br />
imprinting {2243,2334}, supporting the<br />
model that these tumours originate from<br />
an earlier stage of germ cell development<br />
than TGCTs. Although little is known<br />
about the pattern of genomic imprinting<br />
of spermatocytic seminomas {2726} the<br />
available data indicate that these<br />
tumours have already undergone paternal<br />
imprinting.<br />
Testicular germ cell tumours of<br />
adolescents and adults:<br />
Seminomas and non-seminomas<br />
Chromosomal constitution<br />
All TGCTs, including their precursor,<br />
intratubular germ cell neoplasia unclassified<br />
(IGCNU) are aneuploid [{567,676,<br />
1962}, for review]. Seminoma and IGCNU<br />
cells are hypertriploid, while the tumour<br />
cells of non-seminoma, irrespective of<br />
their histological type are hypotriploid.<br />
This suggests that polyploidization is the<br />
initial event, leading to a tetraploid<br />
IGCNU, followed by net loss of chromosomal<br />
material {1962}. Aneuploidy of<br />
TGCTs has been related to the presence<br />
of centrosome amplification {1653}.<br />
224 Tumours of the testis and paratesticular tissue