Polyphasic taxonomy of Penicillium subgenus Penicillium A ... - CBS
Polyphasic taxonomy of Penicillium subgenus Penicillium A ... - CBS
Polyphasic taxonomy of Penicillium subgenus Penicillium A ... - CBS
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
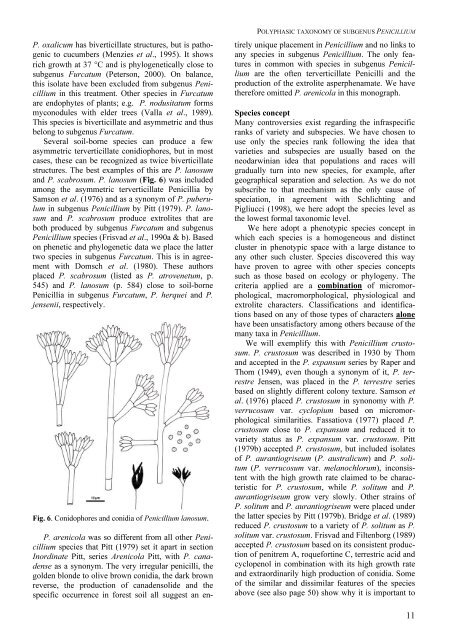
P. oxalicum has biverticillate structures, but is pathogenicto cucumbers (Menzies et al., 1995). It showsrich growth at 37 °C and is phylogenetically close to<strong>subgenus</strong> Furcatum (Peterson, 2000). On balance,this isolate have been excluded from <strong>subgenus</strong> <strong>Penicillium</strong>in this treatment. Other species in Furcatumare endophytes <strong>of</strong> plants; e.g. P. nodusitatum formsmyconodules with elder trees (Valla et al., 1989).This species is biverticillate and asymmetric and thusbelong to <strong>subgenus</strong> Furcatum.Several soil-borne species can produce a fewasymmetric terverticillate conidiophores, but in mostcases, these can be recognized as twice biverticillatestructures. The best examples <strong>of</strong> this are P. lanosumand P. scabrosum. P. lanosum (Fig. 6) was includedamong the asymmetric terverticillate Penicillia bySamson et al. (1976) and as a synonym <strong>of</strong> P. puberulumin <strong>subgenus</strong> <strong>Penicillium</strong> by Pitt (1979). P. lanosumand P. scabrosum produce extrolites that areboth produced by <strong>subgenus</strong> Furcatum and <strong>subgenus</strong><strong>Penicillium</strong> species (Frisvad et al., 1990a & b). Basedon phenetic and phylogenetic data we place the lattertwo species in <strong>subgenus</strong> Furcatum. This is in agreementwith Domsch et al. (1980). These authorsplaced P. scabrosum (listed as P. atrovenetum, p.545) and P. lanosum (p. 584) close to soil-bornePenicillia in <strong>subgenus</strong> Furcatum, P. herquei and P.jensenii, respectively.Fig. 6. Conidophores and conidia <strong>of</strong> <strong>Penicillium</strong> lanosum.POLYPHASIC TAXONOMY OF SUBGENUS PENICILLIUMP. arenicola was so different from all other <strong>Penicillium</strong>species that Pitt (1979) set it apart in sectionInordinate Pitt, series Arenicola Pitt, with P. canadenseas a synonym. The very irregular penicilli, thegolden blonde to olive brown conidia, the dark brownreverse, the production <strong>of</strong> canadensolide and thespecific occurrence in forest soil all suggest an entirelyunique placement in <strong>Penicillium</strong> and no links toany species in <strong>subgenus</strong> <strong>Penicillium</strong>. The only featuresin common with species in <strong>subgenus</strong> <strong>Penicillium</strong>are the <strong>of</strong>ten terverticillate Penicilli and theproduction <strong>of</strong> the extrolite asperphenamate. We havetherefore omitted P. arenicola in this monograph.Species conceptMany controversies exist regarding the infraspecificranks <strong>of</strong> variety and subspecies. We have chosen touse only the species rank following the idea thatvarieties and subspecies are usually based on theneodarwinian idea that populations and races willgradually turn into new species, for example, aftergeographical separation and selection. As we do notsubscribe to that mechanism as the only cause <strong>of</strong>speciation, in agreement with Schlichting andPigliucci (1998), we here adopt the species level asthe lowest formal taxonomic level.We here adopt a phenotypic species concept inwhich each species is a homogeneous and distinctcluster in phenotypic space with a large distance toany other such cluster. Species discovered this wayhave proven to agree with other species conceptssuch as those based on ecology or phylogeny. Thecriteria applied are a combination <strong>of</strong> micromorphological,macromorphological, physiological andextrolite characters. Classifications and identificationsbased on any <strong>of</strong> those types <strong>of</strong> characters alonehave been unsatisfactory among others because <strong>of</strong> themany taxa in <strong>Penicillium</strong>.We will exemplify this with <strong>Penicillium</strong> crustosum.P. crustosum was described in 1930 by Thomand accepted in the P. expansum series by Raper andThom (1949), even though a synonym <strong>of</strong> it, P. terrestreJensen, was placed in the P. terrestre seriesbased on slightly different colony texture. Samson etal. (1976) placed P. crustosum in synonomy with P.verrucosum var. cyclopium based on micromorphologicalsimilarities. Fassatiova (1977) placed P.crustosum close to P. expansum and reduced it tovariety status as P. expansum var. crustosum. Pitt(1979b) accepted P. crustosum, but included isolates<strong>of</strong> P. aurantiogriseum (P. australicum) and P. solitum(P. verrucosum var. melanochlorum), inconsistentwith the high growth rate claimed to be characteristicfor P. crustosum, while P. solitum and P.aurantiogriseum grow very slowly. Other strains <strong>of</strong>P. solitum and P. aurantiogriseum were placed underthe latter species by Pitt (1979b). Bridge et al. (1989)reduced P. crustosum to a variety <strong>of</strong> P. solitum as P.solitum var. crustosum. Frisvad and Filtenborg (1989)accepted P. crustosum based on its consistent production<strong>of</strong> penitrem A, roquefortine C, terrestric acid andcyclopenol in combination with its high growth rateand extraordinarily high production <strong>of</strong> conidia. Some<strong>of</strong> the similar and dissimilar features <strong>of</strong> the speciesabove (see also page 50) show why it is important to11