Proceedings of the European Summer School of Photovoltaics 4 â 7 ...
Proceedings of the European Summer School of Photovoltaics 4 â 7 ...
Proceedings of the European Summer School of Photovoltaics 4 â 7 ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
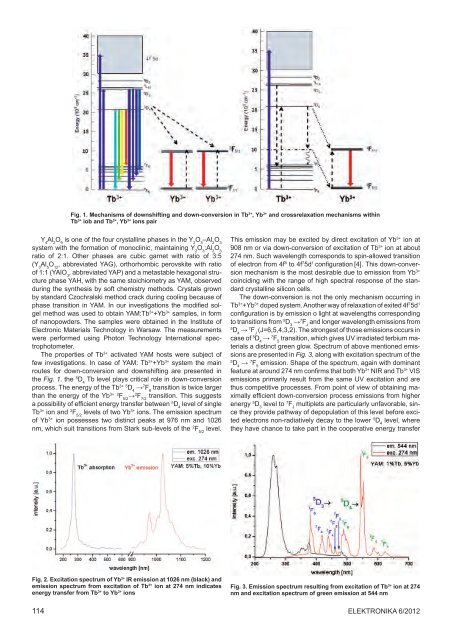
Fig. 1. Mechanisms <strong>of</strong> downshifting and down-conversion in Tb 3+ , Yb 3+ and crossrelaxation mechanisms within<br />
Tb 3+ iob and Tb 3+ , Yb 3+ ions pair<br />
Y 4<br />
Al 2<br />
O 9<br />
is one <strong>of</strong> <strong>the</strong> four crystalline phases in <strong>the</strong> Y 2<br />
O 3<br />
–Al 2<br />
O 3<br />
system with <strong>the</strong> formation <strong>of</strong> monoclinic, maintaining Y 2<br />
O 3<br />
:Al 2<br />
O 3<br />
ratio <strong>of</strong> 2:1. O<strong>the</strong>r phases are cubic garnet with ratio <strong>of</strong> 3:5<br />
(Y 3<br />
Al 5<br />
O 12<br />
, abbreviated YAG), orthorhombic perovskite with ratio<br />
<strong>of</strong> 1:1 (YAlO 3<br />
, abbreviated YAP) and a metastable hexagonal structure<br />
phase YAH, with <strong>the</strong> same stoichiometry as YAM, observed<br />
during <strong>the</strong> syn<strong>the</strong>sis by s<strong>of</strong>t chemistry methods. Crystals grown<br />
by standard Czochralski method crack during cooling because <strong>of</strong><br />
phase transition in YAM. In our investigations <strong>the</strong> modified solgel<br />
method was used to obtain YAM:Tb 3+ +Yb 3+ samples, in form<br />
<strong>of</strong> nanopowders. The samples were obtained in <strong>the</strong> Institute <strong>of</strong><br />
Electronic Materials Technology in Warsaw. The measurements<br />
were performed using Photon Technology International spectrophotometer.<br />
The properties <strong>of</strong> Tb 3+ activated YAM hosts were subject <strong>of</strong><br />
few investigations. In case <strong>of</strong> YAM: Tb 3+ +Yb 3+ system <strong>the</strong> main<br />
routes for down-conversion and downshifting are presented in<br />
<strong>the</strong> Fig. 1. <strong>the</strong> 5 D 4<br />
Tb level plays critical role in down-conversion<br />
process. The energy <strong>of</strong> <strong>the</strong> Tb 3+ 5 D 4<br />
→ 7 F 6<br />
transition is twice larger<br />
than <strong>the</strong> energy <strong>of</strong> <strong>the</strong> Yb 3+ 2 F 5/2<br />
→ 2 F 7/2<br />
transition. This suggests<br />
a possibility <strong>of</strong> efficient energy transfer between 5 D 4<br />
level <strong>of</strong> single<br />
Tb 3+ ion and 2 F 5/2<br />
levels <strong>of</strong> two Yb 3+ ions. The emission spectrum<br />
<strong>of</strong> Yb 3+ ion possesses two distinct peaks at 976 nm and 1026<br />
nm, which suit transitions from Stark sub-levels <strong>of</strong> <strong>the</strong> 2 F 5/2<br />
level.<br />
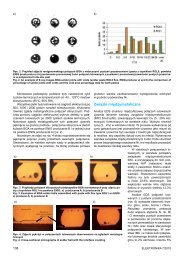
This emission may be excited by direct excitation <strong>of</strong> Yb 3+ ion at<br />
908 nm or via down-conversion <strong>of</strong> excitation <strong>of</strong> Tb 3+ ion at about<br />
274 nm. Such wavelength corresponds to spin-allowed transition<br />
<strong>of</strong> electron from 4f 8 to 4f 7 5d 1 configuration [4]. This down-conversion<br />
mechanism is <strong>the</strong> most desirable due to emission from Yb 3+<br />
coinciding with <strong>the</strong> range <strong>of</strong> high spectral response <strong>of</strong> <strong>the</strong> standard<br />
crystalline silicon cells.<br />
The down-conversion is not <strong>the</strong> only mechanism occurring in<br />
Tb 3+ +Yb 3+ doped system. Ano<strong>the</strong>r way <strong>of</strong> relaxation <strong>of</strong> exited 4f 7 5d 1<br />
configuration is by emission o light at wavelengths corresponding<br />
to transitions from 5 D 3<br />
→ 7 F J<br />
and longer wavelength emissions from<br />
5<br />
D 4<br />
→ 7 F J<br />
(J=6,5,4,3,2). The strongest <strong>of</strong> those emissions occurs in<br />
case <strong>of</strong> 5 D 4<br />
→ 7 F 5<br />
transition, which gives UV irradiated terbium materials<br />
a distinct green glow. Spectrum <strong>of</strong> above mentioned emissions<br />
are presented in Fig. 3, along with excitation spectrum <strong>of</strong> <strong>the</strong><br />
5<br />
D 4<br />
→ 7 F 5<br />
emission. Shape <strong>of</strong> <strong>the</strong> spectrum, again with dominant<br />
feature at around 274 nm confirms that both Yb 3+ NIR and Tb 3+ VIS<br />
emissions primarily result from <strong>the</strong> same UV excitation and are<br />
thus competitive processes. From point <strong>of</strong> view <strong>of</strong> obtaining maximally<br />
efficient down-conversion process emissions from higher<br />
energy 5 D 3<br />
level to 7 F J<br />
multiplets are particularly unfavorable, since<br />
<strong>the</strong>y provide pathway <strong>of</strong> depopulation <strong>of</strong> this level before excited<br />
electrons non-radiatively decay to <strong>the</strong> lower 5 D 4<br />
level, where<br />
<strong>the</strong>y have chance to take part in <strong>the</strong> cooperative energy transfer<br />
Fig. 2. Excitation spectrum <strong>of</strong> Yb 3+ IR emission at 1026 nm (black) and<br />
emission spectrum from excitation <strong>of</strong> Tb 3+ ion at 274 nm indicates<br />
energy transfer from Tb 3+ to Yb 3+ ions<br />
114<br />
Fig. 3. Emission spectrum resulting from excitation <strong>of</strong> Tb 3+ ion at 274<br />
nm and excitation spectrum <strong>of</strong> green emission at 544 nm<br />
Elektronika 6/2012