Proceedings of the European Summer School of Photovoltaics 4 â 7 ...
Proceedings of the European Summer School of Photovoltaics 4 â 7 ...
Proceedings of the European Summer School of Photovoltaics 4 â 7 ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Fig. 2. Changes <strong>of</strong> absorbance at 254 nm <strong>of</strong> HA solution under UV<br />
and ASL irradiation at <strong>the</strong> presence <strong>of</strong> TiO 2<br />
. Concentration <strong>of</strong> photocatalyst:<br />
100 mg l -1 , concentration <strong>of</strong> HA: 40 mg l -1<br />
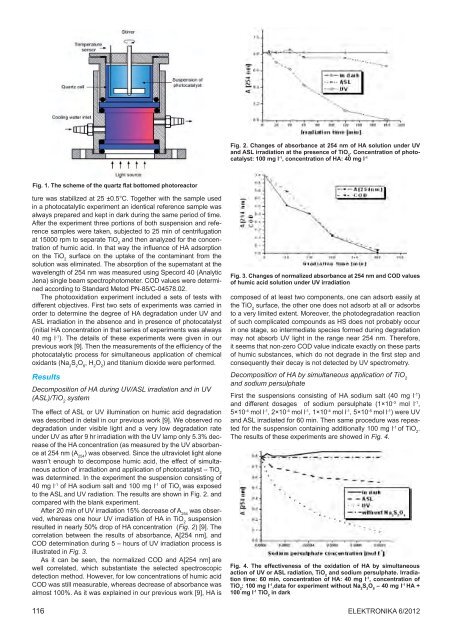
Fig. 1. The scheme <strong>of</strong> <strong>the</strong> quartz flat bottomed photoreactor<br />
ture was stabilized at 25 ±0.5°C. Toge<strong>the</strong>r with <strong>the</strong> sample used<br />
in a photocatalytic experiment an identical reference sample was<br />
always prepared and kept in dark during <strong>the</strong> same period <strong>of</strong> time.<br />
After <strong>the</strong> experiment three portions <strong>of</strong> both suspension and reference<br />
samples were taken, subjected to 25 min <strong>of</strong> centrifugation<br />
at 15000 rpm to separate TiO 2<br />
and <strong>the</strong>n analyzed for <strong>the</strong> concentration<br />
<strong>of</strong> humic acid. In that way <strong>the</strong> influence <strong>of</strong> HA adsorption<br />
on <strong>the</strong> TiO 2<br />
surface on <strong>the</strong> uptake <strong>of</strong> <strong>the</strong> contaminant from <strong>the</strong><br />
solution was eliminated. The absorption <strong>of</strong> <strong>the</strong> supernatant at <strong>the</strong><br />
wavelength <strong>of</strong> 254 nm was measured using Specord 40 (Analytic<br />
Jena) single beam spectrophotometer. COD values were determined<br />
according to Standard Metod PN-85/C-04578.02.<br />
The photooxidation experiment included a sets <strong>of</strong> tests with<br />
different objectives. First two sets <strong>of</strong> experiments was carried in<br />
order to determine <strong>the</strong> degree <strong>of</strong> HA degradation under UV and<br />
ASL irradiation in <strong>the</strong> absence and in presence <strong>of</strong> photocatalyst<br />
(initial HA concentration in that series <strong>of</strong> experiments was always<br />
40 mg l -1 ). The details <strong>of</strong> <strong>the</strong>se experiments were given in our<br />
previous work [9]. Then <strong>the</strong> measurements <strong>of</strong> <strong>the</strong> efficiency <strong>of</strong> <strong>the</strong><br />
photocatalytic process for simultaneous application <strong>of</strong> chemical<br />
oxidants (Na 2<br />
S 2<br />
O 8<br />
, H 2<br />
O 2<br />
) and titanium dioxide were performed.<br />
Results<br />
Decomposition <strong>of</strong> HA during UV/ASL irradiation and in UV<br />
(ASL)/TiO 2<br />
system<br />
The effect <strong>of</strong> ASL or UV illumination on humic acid degradation<br />
was described in detail in our previous work [9]. We observed no<br />
degradation under visible light and a very low degradation rate<br />
under UV as after 9 hr irradiation with <strong>the</strong> UV lamp only 5.3% decrease<br />
<strong>of</strong> <strong>the</strong> HA concentration (as measured by <strong>the</strong> UV absorbance<br />
at 254 nm (A 254<br />
) was observed. Since <strong>the</strong> ultraviolet light alone<br />
wasn’t enough to decompose humic acid, <strong>the</strong> effect <strong>of</strong> simultaneous<br />
action <strong>of</strong> irradiation and application <strong>of</strong> photocatalyst – TiO 2<br />
was determined. In <strong>the</strong> experiment <strong>the</strong> suspension consisting <strong>of</strong><br />
40 mg l -1 <strong>of</strong> HA sodium salt and 100 mg l -1 <strong>of</strong> TiO 2<br />
was exposed<br />
to <strong>the</strong> ASL and UV radiation. The results are shown in Fig. 2. and<br />
compared with <strong>the</strong> blank experiment.<br />
After 20 min <strong>of</strong> UV irradiation 15% decrease <strong>of</strong> A 254<br />
was observed,<br />
whereas one hour UV irradiation <strong>of</strong> HA in TiO 2<br />
suspension<br />
resulted in nearly 50% drop <strong>of</strong> HA concentration (Fig. 2) [9]. The<br />
correlation between <strong>the</strong> results <strong>of</strong> absorbance, A[254 nm], and<br />
COD determination during 5 – hours <strong>of</strong> UV irradiation process is<br />
illustrated in Fig. 3.<br />
As it can be seen, <strong>the</strong> normalized COD and A[254 nm] are<br />
well correlated, which substantiate <strong>the</strong> selected spectroscopic<br />
detection method. However, for low concentrations <strong>of</strong> humic acid<br />
COD was still measurable, whereas decrease <strong>of</strong> absorbance was<br />
almost 100%. As it was explained in our previous work [9], HA is<br />
116<br />
Fig. 3. Changes <strong>of</strong> normalized absorbance at 254 nm and COD values<br />
<strong>of</strong> humic acid solution under UV irradiation<br />
composed <strong>of</strong> at least two components, one can adsorb easily at<br />
<strong>the</strong> TiO 2<br />
surface, <strong>the</strong> o<strong>the</strong>r one does not adsorb at all or adsorbs<br />
to a very limited extent. Moreover, <strong>the</strong> photodegradation reaction<br />
<strong>of</strong> such complicated compounds as HS does not probably occur<br />
in one stage, so intermediate species formed during degradation<br />
may not absorb UV light in <strong>the</strong> range near 254 nm. Therefore,<br />
it seems that non-zero COD value indicate exactly on <strong>the</strong>se parts<br />
<strong>of</strong> humic substances, which do not degrade in <strong>the</strong> first step and<br />
consequently <strong>the</strong>ir decay is not detected by UV spectrometry.<br />
Decomposition <strong>of</strong> HA by simultaneous application <strong>of</strong> TiO 2<br />
and sodium persulphate<br />
First <strong>the</strong> suspensions consisting <strong>of</strong> HA sodium salt (40 mg l -1 )<br />
and different dosages <strong>of</strong> sodium persulphate (1×10 -3 mol l -1 ,<br />
5×10 -4 mol l -1 , 2×10 -4 mol l -1 , 1×10 -4 mol l -1 , 5×10 -5 mol l -1 ) were UV<br />
and ASL irradiated for 60 min. Then same procedure was repeated<br />
for <strong>the</strong> suspension containing additionally 100 mg l -1 <strong>of</strong> TiO 2<br />
.<br />
The results <strong>of</strong> <strong>the</strong>se experiments are showed in Fig. 4.<br />
Fig. 4. The effectiveness <strong>of</strong> <strong>the</strong> oxidation <strong>of</strong> HA by simultaneous<br />
action <strong>of</strong> UV or ASL radiation, TiO 2<br />
and sodium persulphate. Irradiation<br />
time: 60 min, concentration <strong>of</strong> HA: 40 mg l -1 , concentration <strong>of</strong><br />
TiO 2<br />
: 100 mg l -1 ,data for experiment without Na 2<br />
S 2<br />
O 8<br />
– 40 mg l -1 HA +<br />
100 mg l -1 TiO 2<br />
in dark<br />
Elektronika 6/2012