Proceedings of the European Summer School of Photovoltaics 4 â 7 ...
Proceedings of the European Summer School of Photovoltaics 4 â 7 ...
Proceedings of the European Summer School of Photovoltaics 4 â 7 ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Azaheterocyclic materials for organic photovoltaic cells<br />
Natalia Nosidlak 1) , Monika Pokladko-Kowar 1) , Ewa Gondek 1) , Andrzej Danel 2) ,<br />
Jerzy Sanetra 1<br />
1)<br />
Institute <strong>of</strong> Physics, Cracow University <strong>of</strong> Technology, Krakow, Poland<br />
2)<br />
Department <strong>of</strong> Chemistry, University <strong>of</strong> Agriculture, Krakow, Poland<br />
Recently we can observe growing interest in organic photovoltaic<br />
cells. Nowadays, when <strong>the</strong> development <strong>of</strong> renewable sources <strong>of</strong><br />
energy is necessary, photovoltaic energy becomes an opportunity<br />
to reduce consumption <strong>of</strong> fossil fuels. Organic photovoltaic cells<br />
are easy built by deposition techniques like spin coating, moreover<br />
PV devices have lower weight, flexible shape and low production<br />
cost in comparison with inorganic equivalents [1]. These<br />
features have fuelled <strong>the</strong> interest <strong>of</strong> both science and industry.<br />
The conversion <strong>of</strong> solar light into electric power requires <strong>the</strong><br />
generation <strong>of</strong> both negative and positive charges. The operation<br />
<strong>of</strong> a photovoltaic cell can be generally divided into three basic<br />
steps: light absorption, charge separation and charge collection<br />
<strong>of</strong> <strong>the</strong> appropriate carriers to anode and metallic cathode.<br />
(2)<br />
where P light<br />
– power irradiated at cell surface unit [3].<br />
Experiment<br />
For <strong>the</strong> purpose <strong>of</strong> this article <strong>the</strong> regioregular P3OT (poly<br />
3-octylthiophene) (Fig. 2) with HOMO level <strong>of</strong> about – 5,2 eV and<br />
LUMO level about – 2,85 eV, as polymer matrice were choosen.<br />
conversion step<br />
light absorption<br />
exciton creation<br />
exciton diffusion<br />
charge separation<br />
charge transport<br />
charge collection<br />
INCYDENT PHOTONS<br />
reflection and transmission<br />
recombination <strong>of</strong> charges<br />
limited mobility <strong>of</strong> charges<br />
recombination near electrodes<br />
barriers at electrodes<br />
SEPARATED CHARGE AT ELECTRODES<br />
loss mechanism<br />
recombination <strong>of</strong> excitons<br />
exciton transfer<br />
with subsequent recombination <strong>of</strong> excitons<br />
no charge separation<br />
and subsequent recombination <strong>of</strong> excitons<br />
Fig. 1. The specific conversion steps and loss mechanisms in an organic<br />
solar cell<br />
When photons are absorbed, only small part <strong>of</strong> incident light<br />
is absorbed because <strong>of</strong> size <strong>of</strong> band gap. Required band gap is<br />
about 1,1 eV, when most <strong>of</strong> organic semiconductors has 2.0 eV.<br />
This fact reduce possibility <strong>of</strong> absorption to 30%. Also some part<br />
<strong>of</strong> incident light is lost because <strong>of</strong> reflection and transmission.<br />
In case <strong>of</strong> exciton creation and diffusion, loss mechanism includes<br />
recombination <strong>of</strong> excitons. Length <strong>of</strong> diffusion should be<br />
equal to thickness <strong>of</strong> film, but is about 10nm when thickness <strong>of</strong><br />
film is about 100 nm.<br />
In <strong>the</strong> next step <strong>of</strong> conversion loss mechanism includes recombination<br />
<strong>of</strong> excitons but also cases when charges are no<br />
separated. This situation have place when difference between<br />
ionization potential and electron affinity is insufficient and excitons<br />
are able to jump into <strong>the</strong> material with a lower band gap<br />
without charge separation. Charge transport efficiency is limited<br />
by recombination especially when applied material used as transporting<br />
medium is same for electrons and holes. The last step <strong>of</strong><br />
conversion is charge collection on electrodes. Losses here are<br />
caused by recombination near electrodes and by potential barrier<br />
<strong>of</strong> electrodes [2].<br />
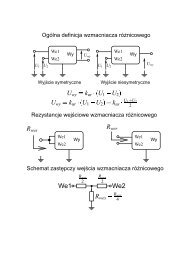
Parameters describing fulfilled solar cells: V OC<br />
– open circuit<br />
voltage, I SC<br />
– short circuit current, I mpp<br />
, V mpp<br />
– voltage and current<br />
at <strong>the</strong> maximum power point, FF – fill factor determined by:<br />
(1)<br />
η – energy conversion efficiency calculated by <strong>the</strong> following formula:<br />
Fig. 2. The principal molecular chemical formula for poly(3-octylthiophene)<br />
The active layer <strong>of</strong> solar cells contain P3OT and three kind<br />
<strong>of</strong> 1H- pyrazolo[3,4-b]quinoxalines: PAQX1, PAQX2, PAQX3,<br />
PAQX4 (Fig. 3).<br />
a) b)<br />
Ph<br />
Ph<br />
N<br />
N<br />
N<br />
N<br />
N<br />
CH 3<br />
c) d)<br />
H 3<br />
C<br />
N C H 3<br />
F N CH 3<br />
M eO N C H 3<br />
N<br />
N<br />
N<br />
Fig. 3. Investigated chromophore molecules <strong>of</strong>: a) PAQX1, b) PAQX2,<br />
c) PAQX4, d) PAQX3<br />
Photovoltaic cells were fabricated on ITO (Indium Tin Oxide)<br />
covered glass slides (15×15 mm) which were cleaned in an ultrasonic<br />
bath using organic solvents. Next <strong>the</strong> ITO was covered<br />
with PEDOT:PSS thin film by spin-coating and left for 30 minutes<br />
in vacuum heater at 70 o C. After 30 minutes <strong>the</strong> active layer<br />
(P3OT + PQXx) was spinned over. At <strong>the</strong> end photovoltaic cells<br />
were defined by aluminum electrodes <strong>the</strong>rmally evaporated in<br />
vacuum. The device has following architecture: ITO/PEDOT:PSS<br />
(poly(3,4-ethylene dioxythiophene)-poly-(styrene sulphonate)/<br />
active layer/Al. Aluminum electrode acts as cathode (collecting<br />
N<br />
N<br />
N<br />
N<br />
N<br />
N<br />
Elektronika 6/2012 129