Proceedings of the European Summer School of Photovoltaics 4 â 7 ...
Proceedings of the European Summer School of Photovoltaics 4 â 7 ...
Proceedings of the European Summer School of Photovoltaics 4 â 7 ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
chanism, as it provides alternative explanation for Yb 3+ emission<br />
under Tb 3+ UV excitation. Due to required multiphonon relaxation<br />
intensity <strong>of</strong> this pathway may be reduced in lower phonon energy<br />
hosts, which will be subject <strong>of</strong> fur<strong>the</strong>r investigations.<br />
Conclusions<br />
Emission <strong>of</strong> NIR light from Yb 3+ ions after excitation <strong>of</strong> <strong>the</strong> Tb 3+<br />
was observed indicating existence <strong>of</strong> transfer mechanisms. Relative<br />
intensities <strong>of</strong> <strong>the</strong>se down-conversion and down-shifting<br />
via single Yb 3+ ion mechanisms is yet to be established. Efficient<br />
down-shifting resulting in visible light emission was observed. Relatively<br />
narrow absorption spectrum with peak at 274 nm limits<br />
potential for application <strong>of</strong> this system in PV systems, due to little<br />
sunlight energy contained within that part <strong>of</strong> <strong>the</strong> solar spectrum.<br />
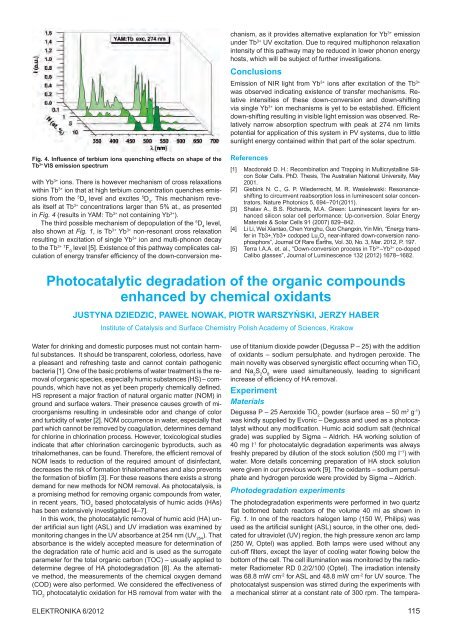
Fig. 4. Influence <strong>of</strong> terbium ions quenching effects on shape <strong>of</strong> <strong>the</strong><br />
Tb 3+ VIS emission spectrum<br />
with Yb 3+ ions. There is however mechanism <strong>of</strong> cross relaxations<br />
within Tb 3+ ion that at high terbium concentration quenches emissions<br />
from <strong>the</strong> 5 D 3<br />
level and excites 5 D 4<br />
. This mechanism reveals<br />
itself at Tb 3+ concentrations larger than 5% at., as presented<br />
in Fig. 4 (results in YAM: Tb 3+ not containing Yb 3+ ).<br />
The third possible mechanism <strong>of</strong> depopulation <strong>of</strong> <strong>the</strong> 5 D 4<br />
level,<br />
also shown at Fig. 1, is Tb 3+ Yb 3+ non-resonant cross relaxation<br />
resulting in excitation <strong>of</strong> single Yb 3+ ion and multi-phonon decay<br />
to <strong>the</strong> Tb 3+ 7 F 0<br />
level [5]. Existence <strong>of</strong> this pathway complicates calculation<br />
<strong>of</strong> energy transfer efficiency <strong>of</strong> <strong>the</strong> down-conversion me-<br />
References<br />
[1] Macdonald D. H.: Recombination and Trapping in Multicrystalline Silicon<br />
Solar Cells. PhD. Thesis, The Australian National University, May<br />
2001.<br />
[2] Giebink N. C., G. P. Wiederrecht, M. R. Wasielewski: Resonanceshifting<br />
to circumvent reabsorption loss in luminescent solar concentrators.<br />
Nature Photonics 5, 694–701(2011).<br />
[3] Shalav A., B.S. Richards, M.A. Green: Luminescent layers for enhanced<br />
silicon solar cell performance: Up-conversion. Solar Energy<br />
Materials & Solar Cells 91 (2007) 829–842.<br />
[4] Li Li, Wei Xiantao, Chen Yonghu, Guo Changxin, Yin Min, “Energy transfer<br />
in Tb3+,Yb3+ codoped Lu 2<br />
O 3<br />
near-infrared down-conversion nanophosphors”,<br />
Journal Of Rare Earths, Vol. 30, No. 3, Mar. 2012, P. 197.<br />
[5] Terra I.A.A. et. al., “Down-conversion process in Tb 3+ –Yb 3+ co-doped<br />
Calibo glasses”, Journal <strong>of</strong> Luminescence 132 (2012) 1678–1682.<br />
Photocatalytic degradation <strong>of</strong> <strong>the</strong> organic compounds<br />
enhanced by chemical oxidants<br />
Justyna Dziedzic, Paweł Nowak, Piotr Warszyński, Jerzy Haber<br />
Institute <strong>of</strong> Catalysis and Surface Chemistry Polish Academy <strong>of</strong> Sciences, Krakow<br />
Water for drinking and domestic purposes must not contain harmful<br />
substances. It should be transparent, colorless, odorless, have<br />
a pleasant and refreshing taste and cannot contain pathogenic<br />
bacteria [1]. One <strong>of</strong> <strong>the</strong> basic problems <strong>of</strong> water treatment is <strong>the</strong> removal<br />
<strong>of</strong> organic species, especially humic substances (HS) – compounds,<br />
which have not as yet been properly chemically defined.<br />
HS represent a major fraction <strong>of</strong> natural organic matter (NOM) in<br />
ground and surface waters. Their presence causes growth <strong>of</strong> microorganisms<br />
resulting in undesirable odor and change <strong>of</strong> color<br />
and turbidity <strong>of</strong> water [2]. NOM occurrence in water, especially that<br />
part which cannot be removed by coagulation, determines demand<br />
for chlorine in chlorination process. However, toxicological studies<br />
indicate that after chlorination carcinogenic byproducts, such as<br />
trihalomethanes, can be found. Therefore, <strong>the</strong> efficient removal <strong>of</strong><br />
NOM leads to reduction <strong>of</strong> <strong>the</strong> required amount <strong>of</strong> disinfectant,<br />
decreases <strong>the</strong> risk <strong>of</strong> formation trihalomethanes and also prevents<br />
<strong>the</strong> formation <strong>of</strong> bi<strong>of</strong>ilm [3]. For <strong>the</strong>se reasons <strong>the</strong>re exists a strong<br />
demand for new methods for NOM removal. As photocatalysis, is<br />
a promising method for removing organic compounds from water,<br />
in recent years, TiO 2<br />
based photocatalysis <strong>of</strong> humic acids (HAs)<br />
has been extensively investigated [4–7].<br />
In this work, <strong>the</strong> photocatalytic removal <strong>of</strong> humic acid (HA) under<br />
artificial sun light (ASL) and UV irradiation was examined by<br />
monitoring changes in <strong>the</strong> UV absorbance at 254 nm (UV 254<br />
). That<br />
absorbance is <strong>the</strong> widely accepted measure for determination <strong>of</strong><br />
<strong>the</strong> degradation rate <strong>of</strong> humic acid and is used as <strong>the</strong> surrogate<br />
parameter for <strong>the</strong> total organic carbon (TOC) – usually applied to<br />
determine degree <strong>of</strong> HA photodegradation [8]. As <strong>the</strong> alternative<br />
method, <strong>the</strong> measurements <strong>of</strong> <strong>the</strong> chemical oxygen demand<br />
(COD) were also performed. We considered <strong>the</strong> effectiveness <strong>of</strong><br />
TiO 2<br />
photocatalytic oxidation for HS removal from water with <strong>the</strong><br />
use <strong>of</strong> titanium dioxide powder (Degussa P – 25) with <strong>the</strong> addition<br />
<strong>of</strong> oxidants – sodium persulphate. and hydrogen peroxide. The<br />
main novelty was observed synergistic effect occurring when TiO 2<br />
and Na 2<br />
S 2<br />
O 8<br />
were used simultaneously, leading to significant<br />
increase <strong>of</strong> efficiency <strong>of</strong> HA removal.<br />
Experiment<br />
Materials<br />
Degussa P – 25 Aeroxide TiO 2<br />
powder (surface area – 50 m 2 g -1 )<br />
was kindly supplied by Evonic – Degussa and used as a photocatalyst<br />
without any modification. Humic acid sodium salt (technical<br />
grade) was supplied by Sigma – Aldrich. HA working solution <strong>of</strong><br />
40 mg l -1 for photocatalytic degradation experiments was always<br />
freshly prepared by dilution <strong>of</strong> <strong>the</strong> stock solution (500 mg l −1 ) with<br />
water. More details concerning preparation <strong>of</strong> HA stock solution<br />
were given in our previous work [9]. The oxidants – sodium persulphate<br />
and hydrogen peroxide were provided by Sigma – Aldrich.<br />
Photodegradation experiments<br />
The photodegradation experiments were performed in two quartz<br />
flat bottomed batch reactors <strong>of</strong> <strong>the</strong> volume 40 ml as shown in<br />
Fig. 1. In one <strong>of</strong> <strong>the</strong> reactors halogen lamp (150 W, Philips) was<br />
used as <strong>the</strong> artificial sunlight (ASL) source, in <strong>the</strong> o<strong>the</strong>r one, dedicated<br />
for ultraviolet (UV) region, <strong>the</strong> high pressure xenon arc lamp<br />
(250 W, Optel) was applied. Both lamps were used without any<br />
cut-<strong>of</strong>f filters, except <strong>the</strong> layer <strong>of</strong> cooling water flowing below <strong>the</strong><br />
bottom <strong>of</strong> <strong>the</strong> cell. The cell illumination was monitored by <strong>the</strong> radiometer<br />
Radiometer RD 0.2/2/100 (Optel). The irradiation intensity<br />
was 68.8 mW cm -2 for ASL and 48.8 mW cm -2 for UV source. The<br />
photocatalyst suspension was stirred during <strong>the</strong> experiments with<br />
a mechanical stirrer at a constant rate <strong>of</strong> 300 rpm. The tempera-<br />
Elektronika 6/2012 115