Set of supplementary notes.
Set of supplementary notes.
Set of supplementary notes.
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
146 CHAPTER 10. FERMI LIQUID THEORY<br />
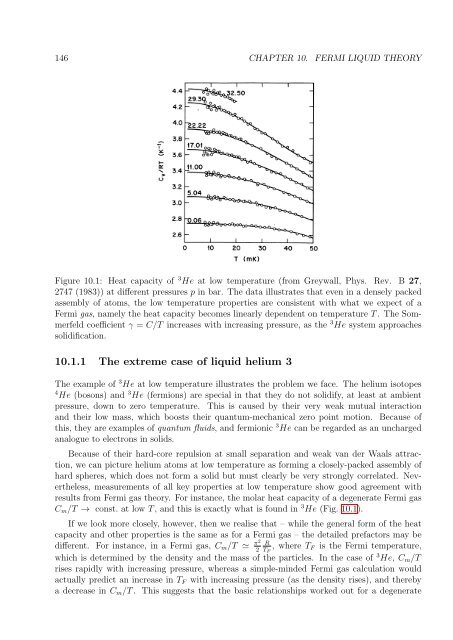
Figure 10.1: Heat capacity <strong>of</strong> 3 He at low temperature (from Greywall, Phys. Rev. B 27,<br />
2747 (1983)) at different pressures p in bar. The data illustrates that even in a densely packed<br />
assembly <strong>of</strong> atoms, the low temperature properties are consistent with what we expect <strong>of</strong> a<br />
Fermi gas, namely the heat capacity becomes linearly dependent on temperature T . The Sommerfeld<br />
coefficient γ = C/T increases with increasing pressure, as the 3 He system approaches<br />
solidification.<br />
10.1.1 The extreme case <strong>of</strong> liquid helium 3<br />
The example <strong>of</strong> 3 He at low temperature illustrates the problem we face. The helium isotopes<br />
4 He (bosons) and 3 He (fermions) are special in that they do not solidify, at least at ambient<br />
pressure, down to zero temperature. This is caused by their very weak mutual interaction<br />
and their low mass, which boosts their quantum-mechanical zero point motion. Because <strong>of</strong><br />
this, they are examples <strong>of</strong> quantum fluids, and fermionic 3 He can be regarded as an uncharged<br />
analogue to electrons in solids.<br />
Because <strong>of</strong> their hard-core repulsion at small separation and weak van der Waals attraction,<br />
we can picture helium atoms at low temperature as forming a closely-packed assembly <strong>of</strong><br />
hard spheres, which does not form a solid but must clearly be very strongly correlated. Nevertheless,<br />
measurements <strong>of</strong> all key properties at low temperature show good agreement with<br />
results from Fermi gas theory. For instance, the molar heat capacity <strong>of</strong> a degenerate Fermi gas<br />
C m /T → const. at low T , and this is exactly what is found in 3 He (Fig. 10.1).<br />
If we look more closely, however, then we realise that – while the general form <strong>of</strong> the heat<br />
capacity and other properties is the same as for a Fermi gas – the detailed prefactors may be<br />
different. For instance, in a Fermi gas, C m /T ≃ π2 R<br />
2 T F<br />
, where T F is the Fermi temperature,<br />
which is determined by the density and the mass <strong>of</strong> the particles. In the case <strong>of</strong> 3 He, C m /T<br />
rises rapidly with increasing pressure, whereas a simple-minded Fermi gas calculation would<br />
actually predict an increase in T F with increasing pressure (as the density rises), and thereby<br />
a decrease in C m /T . This suggests that the basic relationships worked out for a degenerate