Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
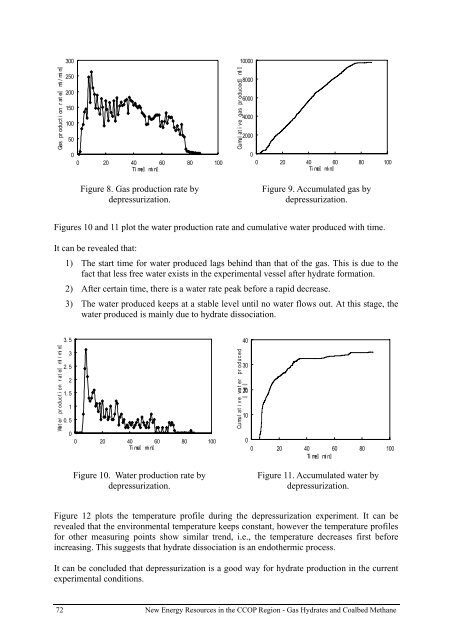
Gas pr oduct i on r at e ml /min<br />
300<br />
250<br />
200<br />
150<br />
100<br />
50<br />
0<br />
0 20 40 60 80 100<br />
Ti me<br />
min<br />
Figure 8. Gas production rate by<br />
depressurization.<br />
<br />
Cumul at i ve <strong>gas</strong> pr oduced ml<br />
10000<br />
8000<br />
6000<br />
4000<br />
2000<br />
0<br />
0 20 40 60 80 100<br />
Ti me<br />
min<br />
Figure 9. Accumulated <strong>gas</strong> by<br />
depressurization.<br />
Figures 10 and 11 plot the water production rate and cumulative water produced with time.<br />
It can be revealed that:<br />
1) The start time for water produced lags behind than that of the <strong>gas</strong>. This is due to the<br />
fact that less free water exists in the experimental vessel after <strong>hydrate</strong> formation.<br />
2) After certain time, there is a water rate peak before a rapid decrease.<br />
3) The water produced keeps at a stable level until no water flows out. At this stage, the<br />
water produced is mainly due to <strong>hydrate</strong> dissociation.<br />
<br />
Wat er pr oduct i on r at e ml / mi n<br />
3. 5<br />
3<br />
2. 5<br />
2<br />
1. 5<br />
1<br />
0. 5<br />
0<br />
0 20 40 60 80 100<br />
Ti me<br />
min<br />
Figure 10. Water production rate by<br />
depressurization.<br />
40<br />
Cumul at i ve wat er pr oduced<br />
ml <br />
30<br />
20<br />
10<br />
0<br />
0 20 40 60 80 100<br />
Ti me<br />
min<br />
Figure 11. Accumulated water by<br />
depressurization.<br />
Figure 12 plots the temperature profile during the depressurization experiment. It can be<br />
revealed that the environmental temperature keeps constant, however the temperature profiles<br />
for other measuring points show similar trend, i.e., the temperature decreases first before<br />
increasing. This suggests that <strong>hydrate</strong> dissociation is an endothermic process.<br />
It can be concluded that depressurization is a good way for <strong>hydrate</strong> production in the current<br />
experimental conditions.<br />
72<br />
New Energy Resources in the <strong>CCOP</strong> Region - Gas Hydrates and Coalbed Methane