Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
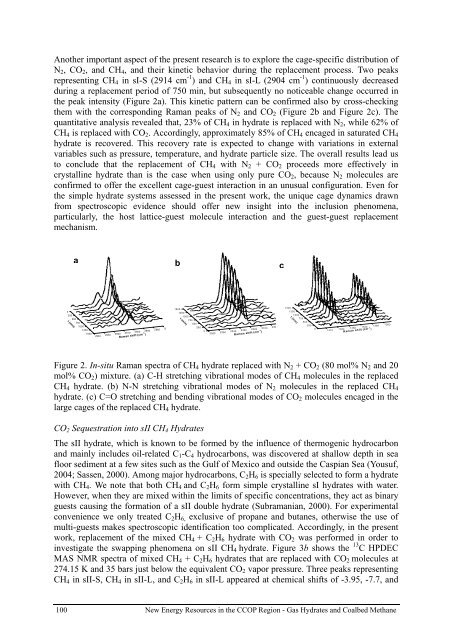
Another important aspect of the present research is to explore the cage-specific distribution of<br />
N 2 , CO 2 , and CH 4 , and their kinetic behavior during the replacement process. Two peaks<br />
representing CH 4 in sI-S (2914 cm -1 ) and CH 4 in sI-L (2904 cm -1 ) continuously decreased<br />
during a replacement period of 750 min, but subsequently no noticeable change occurred in<br />
the peak intensity (Figure 2a). This kinetic pattern can be confirmed also by cross-checking<br />
them with the corresponding Raman peaks of N 2 and CO 2 (Figure 2b and Figure 2c). The<br />
quantitative analysis revealed that, 23% of CH 4 in <strong>hydrate</strong> is replaced with N 2 , while 62% of<br />
CH 4 is replaced with CO 2 . Accordingly, approximately 85% of CH 4 encaged in saturated CH 4<br />
<strong>hydrate</strong> is recovered. This recovery rate is expected to change with variations in external<br />
variables such as pressure, temperature, and <strong>hydrate</strong> particle size. The overall results lead us<br />
to conclude that the replacement of CH 4 with N 2 + CO 2 proceeds more effectively in<br />
crystalline <strong>hydrate</strong> than is the case when using only pure CO 2 , because N 2 molecules are<br />
confirmed to offer the excellent cage-guest interaction in an unusual configuration. Even for<br />
the simple <strong>hydrate</strong> systems assessed in the present work, the unique cage dynamics drawn<br />
from spectroscopic evidence should offer new insight into the inclusion phenomena,<br />
particularly, the host lattice-guest molecule interaction and the guest-guest replacement<br />
mechanism.<br />
a b c<br />
0<br />
250<br />
500<br />
t (min)<br />
750<br />
1000<br />
1250<br />
1500<br />
2940<br />
2930<br />
2920<br />
2910<br />
2900<br />
2890<br />
Raman shift (cm -1 )<br />
2880<br />
2870<br />
1500<br />
1250<br />
1000<br />
750<br />
t (min)<br />
500<br />
250<br />
0<br />
2360<br />
2350<br />
2340<br />
2330<br />
2320<br />
2310<br />
Ram an shift (cm -1 )<br />
2300<br />
1500<br />
1250<br />
1000<br />
750<br />
t (min)<br />
500<br />
250<br />
0<br />
1410<br />
1380<br />
1350<br />
1290<br />
1320<br />
R am an sh ift (cm -1 )<br />
1260<br />
Figure 2. In-situ Raman spectra of CH 4 <strong>hydrate</strong> replaced with N 2 + CO 2 (80 mol% N 2 and 20<br />
mol% CO 2 ) mixture. (a) C-H stretching vibrational modes of CH 4 molecules in the replaced<br />
CH 4 <strong>hydrate</strong>. (b) N-N stretching vibrational modes of N 2 molecules in the replaced CH 4<br />
<strong>hydrate</strong>. (c) C=O stretching and bending vibrational modes of CO 2 molecules encaged in the<br />
large cages of the replaced CH 4 <strong>hydrate</strong>.<br />
CO 2 Sequestration into sII CH 4 Hydrates<br />
The sII <strong>hydrate</strong>, which is known to be formed by the influence of thermogenic hydrocarbon<br />
and mainly includes oil-related C 1 -C 4 hydrocarbons, was discovered at shallow depth in sea<br />
floor sediment at a few sites such as the Gulf of Mexico and outside the Caspian Sea (Yousuf,<br />
2004; Sassen, 2000). Among major hydrocarbons, C 2 H 6 is specially selected to form a <strong>hydrate</strong><br />
with CH 4 . We note that both CH 4 and C 2 H 6 form simple crystalline sI <strong>hydrate</strong>s with water.<br />
However, when they are mixed within the limits of specific concentrations, they act as binary<br />
guests causing the formation of a sII double <strong>hydrate</strong> (Subramanian, 2000). For experimental<br />
convenience we only treated C 2 H 6, exclusive of propane and butanes, otherwise the use of<br />
multi-guests makes spectroscopic identification too complicated. Accordingly, in the present<br />
work, replacement of the mixed CH 4 + C 2 H 6 <strong>hydrate</strong> with CO 2 was performed in order to<br />
investigate the swapping phenomena on sII CH 4 <strong>hydrate</strong>. Figure 3b shows the 13 C HPDEC<br />
MAS NMR spectra of mixed CH 4 + C 2 H 6 <strong>hydrate</strong>s that are replaced with CO 2 molecules at<br />
274.15 K and 35 bars just below the equivalent CO 2 vapor pressure. Three peaks representing<br />
CH 4 in sII-S, CH 4 in sII-L, and C 2 H 6 in sII-L appeared at chemical shifts of -3.95, -7.7, and<br />
100<br />
New Energy Resources in the <strong>CCOP</strong> Region - Gas Hydrates and Coalbed Methane