You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
same crystalline structure directly after its replacement with CO 2, this not only enables the<br />
ocean floor to remain stable even after recovering the CH 4 <strong>gas</strong>, but also makes the swapping<br />
process more viable by enhancing its economical efficiency.<br />
Although numerous <strong>hydrate</strong> studies, involving both macroscopic and microscopic approaches,<br />
have recently been conducted for a variety of purposes, and to a certain extent have yielded<br />
notable success, little attention has been paid to cage dynamics in exploring guest<br />
distributions within the sensitive host-guest networks. Moreover, the complex <strong>hydrate</strong><br />
behavior occurring under strong attacks by external guest molecules on the existing cages has<br />
not yet been fully considered, and no detailed study exists even at a very fundamental level. In<br />
a previous study, we explored the replacement mechanism of CH 4 <strong>hydrate</strong> with CO 2 using<br />
spectroscopic methods and found that when a CH 4 <strong>hydrate</strong> is exposed to <strong>gas</strong> mixtures<br />
containing CO 2 , CH 4 is replaced by CO 2 in mainly the large cages (Lee, 2003). If the CH 4<br />
<strong>hydrate</strong>s could be converted into CO 2 <strong>hydrate</strong>s, they would serve doubly as CH 4 sources and<br />
CO 2 storage sites. Here, we further extend our investigations to consider the occurrence of<br />
CO 2 replacement phenomena on sII <strong>hydrate</strong>, which is thought to exist in the seabed. From this<br />
point of view, we present an interesting conclusion reached by inducing a structure transition.<br />
A microscopic analysis is conducted in order to examine the real swapping phenomena<br />
occurring between CO 2 guest molecules and sII <strong>hydrate</strong> through spectroscopic identification,<br />
including solid-state Nuclear Magnetic Resonance (NMR) spectrometry and FT-Raman<br />
spectrometry. More importantly, we also investigate the possibility of the direct use of binary<br />
N 2 and CO 2 <strong>gas</strong> mixture for recovering CH 4 from the <strong>hydrate</strong> phase, which shows a<br />
remarkably enhanced recovery rate by means of the cage-specific occupation of guest<br />
molecules due to their molecular properties.<br />
RESULTS AND DISCUSSION<br />
Direct sequestration of CO 2 and N 2 Mixtures into sI CH 4 <strong>hydrate</strong>s<br />
In the preceding work we verified that the CH 4 amount that could be recovered by replacing<br />
sI CH 4 <strong>hydrate</strong> with CO 2 could reach around 64% of the <strong>hydrate</strong> composition. CO 2 molecules<br />
only preferably replaced CH 4 in large cages, while CH 4 molecules in small cages remain<br />
almost intact (Lee, 2003). Due to such a preferential cage occupation of guest molecules, the<br />
recovery rate of CH 4 is limited to the maximum value of 64 %.<br />
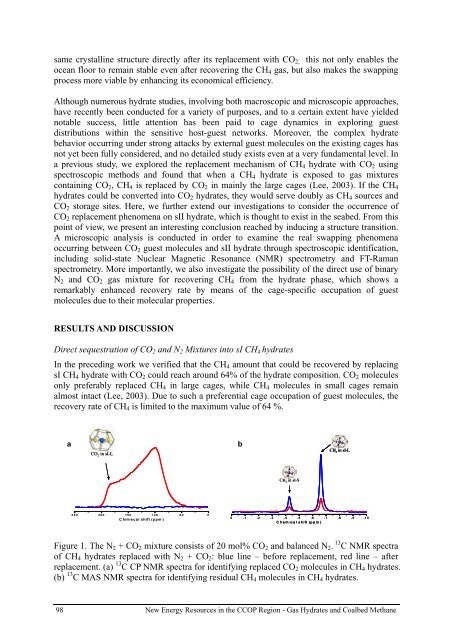
a<br />
CO 2 in sI-L<br />
b<br />
CH 4 in sI-L<br />
CH 4 in sI-S<br />
250 200 150 100 50 0<br />
Chimecal shift (ppm)<br />
0 -1 -2 -3 -4 -5 -6 -7 -8 -9 -10<br />
Chem ical shift (ppm )<br />
Figure 1. The N 2 + CO 2 mixture consists of 20 mol% CO 2 and balanced N 2 . 13 C NMR spectra<br />
of CH 4 <strong>hydrate</strong>s replaced with N 2 + CO 2 : blue line – before replacement, red line – after<br />
replacement. (a) 13 C CP NMR spectra for identifying replaced CO 2 molecules in CH 4 <strong>hydrate</strong>s.<br />
(b) 13 C MAS NMR spectra for identifying residual CH 4 molecules in CH 4 <strong>hydrate</strong>s.<br />
98<br />
New Energy Resources in the <strong>CCOP</strong> Region - Gas Hydrates and Coalbed Methane