Combining health and social protection measures to reach the ultra ...
Combining health and social protection measures to reach the ultra ...
Combining health and social protection measures to reach the ultra ...
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Access <strong>to</strong> <strong>health</strong>threatening <strong>and</strong> serious conditions <strong>to</strong> be registered on <strong>the</strong>ClinicalTrials.gov databank, detailed analyses indicate that asignificant number of industry-sponsors do not comply with<strong>the</strong> requirement 41 .To support WHO’s initiative, funding agencies, academiccentres, philanthropic organizations, <strong>and</strong> supportive industrialsponsors ought <strong>to</strong> develop strict compliance mechanisms.Registration should be imposed as a requirement for researchethics approval. Patient advocacy groups could attachregistration as a condition <strong>to</strong> support <strong>and</strong> advertise clinicaltrials. The best approach would be for domestic governmentswith control over existing primary registries <strong>to</strong> endow relevantgoverning bodies with <strong>the</strong> necessary legal jurisdiction <strong>to</strong> enforcecompliance through sanctions. Given industry’s concern aboutcompetitive advantage, it seems particularly important <strong>to</strong>assure pharmaceutical companies that <strong>the</strong>re is a level playingfield, by ensuring compliance with registration requirements.Domestic regulations <strong>and</strong> state control can do that.Even though <strong>the</strong> WHO minimal data set extends wellbeyond information required <strong>to</strong> be registered in several o<strong>the</strong>rregistries, some have criticized <strong>the</strong> WHO initiative for providinginsufficient information. The Ottawa Statement, for example,proposes that registration data fur<strong>the</strong>r include information on<strong>the</strong> full pro<strong>to</strong>col <strong>and</strong> consent forms, details of ethics committeeapproval <strong>and</strong> o<strong>the</strong>r trial design information 42 .Registration of <strong>the</strong> WHO data set will indeed not resolve allproblems associated with manipulation of trial results 43 . It isunclear how <strong>the</strong> accuracy of registration data that are not peerreviewedas well as <strong>the</strong> scientific validity of statistical analyses<strong>and</strong> interpretations could be validated using information fromexisting registries or resources. The registration system doesnot alleviate concerns associated with <strong>the</strong> fact that sponsorswith vested commercial interests still control how research isdesigned, how subjects are recruited, how data are collected,<strong>and</strong> how results are presented 44 . The data set does not provideregistry staff or researchers with access <strong>to</strong> pro<strong>to</strong>cols or rawdata, which significantly hampers independent scientificreview of database entries. Without <strong>the</strong> ability <strong>to</strong>independently review <strong>and</strong> validate entries, selective reportingof trial results may still occur. Finally, <strong>the</strong> basic principles ofevidence-based medicine require review of study design <strong>and</strong>quality in addition <strong>to</strong> o<strong>the</strong>r relevant scientific data prior <strong>to</strong>drawing conclusions from a single study 45 .Finally, WHO’s clinical trials registration system does notimpose results reporting. It allows <strong>the</strong> public, researchers, <strong>and</strong>governmental agencies <strong>to</strong> know that research is or has beenundertaken, which allows for fur<strong>the</strong>r scrutiny <strong>and</strong> questionswhen publications come out. But it does not provide <strong>the</strong>mwith direct access <strong>to</strong> final outcomes. Several influentialorganizations have called for public disclosure of results in amanner analogous <strong>to</strong> clinical trial registration, including <strong>the</strong>US Institute of Medicine, ICMJE, <strong>and</strong> researchers 46 . As notedby <strong>the</strong> ICMJE in <strong>the</strong>ir most recent discussion statement onm<strong>and</strong>a<strong>to</strong>ry registration 47 , <strong>the</strong> climate for results registration willlikely change dramatically <strong>and</strong> unpredictably over comingyears but is sure <strong>to</strong> go ahead in some form.WHO has recognized that this is a next important step in <strong>the</strong>promotion of research integrity. It has set up a Study Group on<strong>the</strong> Reporting of Findings of Clinical Trials, which is looking at<strong>the</strong> development of criteria <strong>and</strong> st<strong>and</strong>ards of disclosure ofresults 48 . This new initiative will constitute ano<strong>the</strong>r importantstep <strong>to</strong>wards more reliable <strong>and</strong> trustworthy clinical research.Result reporting will only have success, however, if a coherent,comprehensive, <strong>and</strong> m<strong>and</strong>a<strong>to</strong>ry registration system is in place.It seems <strong>the</strong>refore crucial that national governments,professional organizations, industry, <strong>and</strong> researchers commit<strong>to</strong> such a registration system <strong>and</strong> fully support WHO in itsefforts. ❏Trudo Lemmens is an associate professor at <strong>the</strong> Faculties of Law<strong>and</strong> Medicine of <strong>the</strong> University of Toron<strong>to</strong>. His research currentlyfocuses on how law <strong>and</strong> regulation contribute <strong>to</strong> <strong>the</strong> promotion ofethical st<strong>and</strong>ards in <strong>the</strong> context of medical research <strong>and</strong>biotechnological innovations. Recent publications include <strong>the</strong> coeditedvolume Law <strong>and</strong> Ethics in Biomedical Research: Regulation,Conflict of Interest, <strong>and</strong> Liability <strong>and</strong> <strong>the</strong> co-authored book Reading<strong>the</strong> future? Legal <strong>and</strong> Ethical Challenges of Predictive GeneticTesting. In <strong>the</strong> last four years, Trudo Lemmens has been a memberof <strong>the</strong> Institute for Advanced Studies in Prince<strong>to</strong>n, a visitingprofessor at <strong>the</strong> KU Leuven <strong>and</strong> <strong>the</strong> University of Otago, <strong>and</strong> aFellow of <strong>the</strong> Royal Flemish Academy of Belgium for Science <strong>and</strong><strong>the</strong> Arts. Since 2006, he has been a member of <strong>the</strong> PAHO AdvisoryCommittee on Health Research.Ron A Bouchard is a doc<strong>to</strong>ral c<strong>and</strong>idate in law <strong>and</strong> runs his ownconsulting firm. His career has focused on <strong>the</strong> science, law <strong>and</strong>policy of pharmaceuticals <strong>and</strong> biotechnology as well as strategicplanning for commercialization of innovative technologies. Hebegan his career as a scientist, obtaining a doc<strong>to</strong>rate <strong>and</strong> workingin <strong>the</strong> field of membrane electrophysiology. He shifted focus <strong>to</strong> law,<strong>and</strong> has been involved in <strong>the</strong> prosecution, acquisition, financing,distribution, <strong>and</strong> litigation of intellectual property rights relating <strong>to</strong>pharmaceuticals <strong>and</strong> biotechnology. He has appeared before <strong>the</strong>Federal Court of Canada on trial <strong>and</strong> appeal matters <strong>and</strong> <strong>the</strong>Supreme Court of Canada. He currently conducts research on drugregulation <strong>and</strong> innovation from <strong>the</strong> perspective of systems dynamics<strong>and</strong> complex adaptive systems.AcknowledgementsResearch for this chapter was funded by Genome Canada through<strong>the</strong> Ontario Genomics Institute, by Génome Québec, <strong>the</strong> Ministère duDéveloppement Économique et Régional et de la Recherche duQuébec <strong>and</strong> <strong>the</strong> Ontario Cancer Research Network, as part of <strong>the</strong>ARCTIC project. Ron Bouchard also received support from <strong>the</strong> CIHRHealth Law & Policy Program <strong>and</strong> <strong>the</strong> Lupina FoundationComparative Program in Health <strong>and</strong> Society at <strong>the</strong> Munk Centre forInternational Studies. The authors thank Michelle Jackson for work on<strong>the</strong> references.Global Forum Update on Research for Health Volume 4 ✜ 043














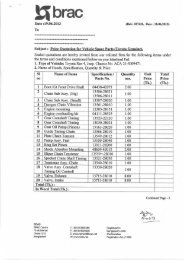
![[re-tender] RFQ for supply of Diesel Generator - Brac](https://img.yumpu.com/44421374/1/186x260/re-tender-rfq-for-supply-of-diesel-generator-brac.jpg?quality=85)
