Combining health and social protection measures to reach the ultra ...
Combining health and social protection measures to reach the ultra ...
Combining health and social protection measures to reach the ultra ...
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Access <strong>to</strong> <strong>health</strong>References1.World Health Organization (WHO). Ministerial Summit on HealthResearch, 2004. Online: http://www.who.int/rpc/summit/en/ (dateaccessed 21 July 2007).2.World Health Organization (WHO) (A). International Clinical Trials RegistryPlatform. Online: http://www.who.int/ictrp/about/en/ (date accessed July21 2007).3.Symes RJ. Publication bias: <strong>the</strong> case for an international registry of clinicaltrials. Journal of Clinical Oncology, 1986, vol. 4, pp. 1529-1541.Dickersin K. Report from <strong>the</strong> panel on <strong>the</strong> case for registers of clinicaltrials at <strong>the</strong> eighth annual meeting of <strong>the</strong> society for clinical trials.Controlled Clinical Trials, 1988, vol. 9 pp. 76-81. Dickersin K & RennieD. Registering clinical trials. Journal of <strong>the</strong> American MedicalAssociation, 2003, vol. 290, no. 4, pp. 516-523.4.Rennie D. Trial registration: a great idea switches from ignored <strong>to</strong>irresistible. Journal of <strong>the</strong> American Medical Association, 2004, vol.292, pp. 1359-1362. Lemmens T. Leopards in <strong>the</strong> temple: res<strong>to</strong>ringintegrity <strong>to</strong> <strong>the</strong> commercialized research scene. Journal of Law, Medicine& Ethics, 2005, vol. 32, no. 4, pp. 641-657.5.Angell M. The truth about <strong>the</strong> drug companies: how <strong>the</strong>y deceive us <strong>and</strong>what <strong>to</strong> do about it. R<strong>and</strong>om House, New York, 2004. Dickersin K &Rennie D. Registering clinical trials. Journal of <strong>the</strong> American MedicalAssociation, 2003, vol. 290, no. 4, pp. 516-523. Kassirer J. On <strong>the</strong>take: how medicine’s complicity with big business can endanger your<strong>health</strong>. Oxford University Press, New York, 2004.6.Rennie D. Trial registration: a great idea switches from ignored <strong>to</strong>irresistible. Journal of <strong>the</strong> American Medical Association, 2004, vol.292, pp. 1359-1362.7.Office of <strong>the</strong> New York State At<strong>to</strong>rney General. Settlement sets newst<strong>and</strong>ard for release of drug information: Glaxo <strong>to</strong> establish “clinical trialsregister” with information on all company drugs, 2004. Online:http://www.oag.state.ny.us/press/2004/aug/aug26a_04.html (dateaccessed 20 May 2007).8.Rennie D. Trial registration: a great idea switches from ignored <strong>to</strong>irresistible. Journal of <strong>the</strong> American Medical Association, 2004, vol.292, pp. 1359-1362.9.Krleza-Jeric K et al. Principles for international registration of pro<strong>to</strong>colinformation <strong>and</strong> results from human trials of <strong>health</strong> related interventions:Ottawa Statement (part 1). British Medical Journal, 2005, vol. 330, pp.956-958.10.De Angelis CD et al. Clinical trial registration: a statement from <strong>the</strong>International Committee of Medical Journal Edi<strong>to</strong>rs. New Engl<strong>and</strong> Journalof Medicine, 2004, vol. 351, no.12, pp. 1250-1251.11.Laine C et al. Clinical trial registration: Looking back <strong>and</strong> moving ahead.New Engl<strong>and</strong> Journal of Medicine, 2007, vol. 356, no. 26, pp. 2734-2733.12.Sim I et al. Clinical trial registration: transparency is <strong>the</strong> watchword. TheLancet, 2006, vol. 367, no.9523, pp. 1631-1633.13.International Federation of Pharmaceutical Manufacturers & Associates.International Clinical Trials Registry Platform (ICTRP): IFPMA comments<strong>to</strong> ICTRP consultation on delayed disclosure, 2006. Online:www.who.int/entity/ictrp/3005_IFPMA_25Jan06.pdf (date accessed 21July 2007). Krall R & Rockhold F. More on compulsory registration ofclinical trials: GSK has created useful register. British Medical Journal,2005, vol. 330, pp. 479–480. Krall RL & Rockhold F. Trial registration:Putting principles in<strong>to</strong> practice, 2005b. Online:http://bmj.bmjjournals.com/cgi/eletters/330/7497/956#107176(accessed 21 July 2007).14.Krleza-Jeric K. Clinical trial registration: <strong>the</strong> differing views of industry,<strong>the</strong> WHO <strong>and</strong> <strong>the</strong> Ottawa Group. PLoS Medicine, 2005, vol. 2 no.11,pp. 1098-1097.15.De Angelis CD et al. Clinical trial registration: a statement from <strong>the</strong>International Committee of Medical Journal Edi<strong>to</strong>rs. New Engl<strong>and</strong> Journalof Medicine, 2004, vol. 351, no.12, pp. 1250-1251.16.Lemmens T. Leopards in <strong>the</strong> temple: res<strong>to</strong>ring integrity <strong>to</strong> <strong>the</strong>commercialized research scene. Journal of Law, Medicine & Ethics,2005, vol. 32, no. 4, pp. 641-657. Bouchard RA. Balancing public <strong>and</strong>private interests in commercialization of publicly funded biomedicaltechnologies: Is <strong>the</strong>re a role for compulsory government royalty fees?.Bos<strong>to</strong>n University Journal of Science <strong>and</strong> Technology Law, 2007, vol.13, no. 2. (forthcoming).17.Godlee F. Winning hearts <strong>and</strong> minds. British Medical Journal, 2005, vol.330, pp.1224. Bur<strong>to</strong>n B. Drug chiefs ponder how <strong>to</strong> improve industry’sreputation. British Medical Journal, 2005, vol. 330, pp. 122918.Kahn JP. Beyond disclosure: <strong>the</strong> necessity of trust in biomedical research.Clevel<strong>and</strong> Clinic Journal of Medicine, 2007, vol. 74(S2): S49-S50. IrwinRS. Clinical trial registration promotes patient <strong>protection</strong> <strong>and</strong> benefit,advances <strong>the</strong> trust of everyone, <strong>and</strong> is required. Chest, 2007, vol. 131,no. 3, pp. 639-641.19.Chalmers I. Underreporting research is scientific misconduct. Journal of<strong>the</strong> American Medical Association, 1990, vol. 263, no. 10, pp. 1405-1408. De Angelis CD et al. Clinical trial registration: a statement from <strong>the</strong>International Committee of Medical Journal Edi<strong>to</strong>rs. New Engl<strong>and</strong> Journalof Medicine, 2004, vol. 351, no.12, pp. 1250-1251.20.World Medical Association. Declaration of Helsinki, 1964 (last update2000). Online: http://www.wma.net/e/policy/b3.htm (date accessed 21July 2007).21.The Nuremburg Code. Trials of War Criminals before <strong>the</strong> NurembergMilitary Tribunals under Control Council Law, 1949, No. 10, Vol. 2, pp.181-182. Washing<strong>to</strong>n, DC: US Government Printing Office. Onlinehttp://www.hhs.gov/ohrp/references/nurcode.htm (date accessed 21 July2007).22.Sim I et al. Clinical trial registration: transparency is <strong>the</strong> watchword. TheLancet, 2006, vol. 367, no.9523, pp. 1631-1633.23.De Angelis CD et al. Is this trial fully registered? A statement from <strong>the</strong>International Committee of Medical Journal Edi<strong>to</strong>rs. New Engl<strong>and</strong> Journalof Medicine, 2005, vol. 352, no.23, pp. 2436-2438.24.Tonks A. Registering clinical trials. British Medical Journal, 1999, vol.319, no. 7224, pp. 1565-1568.25.Chalmers TC. R<strong>and</strong>omize <strong>the</strong> first patient! New Engl<strong>and</strong> Journal ofMedicine, 1977, vol. 296, pp. 107. Dickersin K & Rennie D. Registeringclinical trials. Journal of <strong>the</strong> American Medical Association, 2003, vol.290, no. 4, pp. 516-523.26.Tonks A. Registering clinical trials. British Medical Journal, 1999, vol.319, no. 7224, pp. 1565-1568.27.Krleza-Jeric K. Clinical trial registration: <strong>the</strong> differing views of industry,<strong>the</strong> WHO <strong>and</strong> <strong>the</strong> Ottawa Group. PLoS Medicine, 2005, vol. 2 no.11,pp. 1098-1097.28.Tonks A. Registering clinical trials. British Medical Journal, 1999, vol.319, no. 7224, pp. 1565-1568.29.Dickersin K & Rennie D. Registering clinical trials. Journal of <strong>the</strong>American Medical Association, 2003, vol. 290, no. 4, pp. 516-523. SimI et al. Clinical trial registration: transparency is <strong>the</strong> watchword. TheLancet, 2006, vol. 367, no.9523, pp. 1631-1633. Irwin RS. Clinicaltrial registration promotes patient <strong>protection</strong> <strong>and</strong> benefit, advances <strong>the</strong>trust of everyone, <strong>and</strong> is required. Chest, 2007, vol. 131, no. 3, pp.639-641. Chan A-W et al. Empirical evidence for selective reporting ofoutcomes in r<strong>and</strong>omized clinical trials. Comparison of pro<strong>to</strong>cols <strong>to</strong>published articles. Journal of <strong>the</strong> American Medical Association, 2005,vol. 291, no. 20, pp. 2457-2465.30.Dickersin K & Rennie D. Registering clinical trials. Journal of <strong>the</strong>American Medical Association, 2003, vol. 290, no. 4, pp. 516-523.31.Sim I et al. Clinical trial registration: transparency is <strong>the</strong> watchword. TheLancet, 2006, vol. 367, no.9523, pp. 1631-1633. Krleza-Jeric K.Clinical trial registration: <strong>the</strong> differing views of industry, <strong>the</strong> WHO <strong>and</strong> <strong>the</strong>Ottawa Group. PLoS Medicine, 2005, vol. 2 no.11, pp. 1098-1097.32.Tonks A. Registering clinical trials. British Medical Journal, 1999, vol.319, no. 7224, pp. 1565-1568.33.Tonks A. Registering clinical trials. British Medical Journal, 1999, vol.319, no. 7224, pp. 1565-1568.34.Tonks A. Registering clinical trials. British Medical Journal, 1999, vol.319, no. 7224, pp. 1565-1568.35.Sim I et al. Clinical trial registration: transparency is <strong>the</strong> watchword. TheGlobal Forum Update on Research for Health Volume 4 ✜ 045














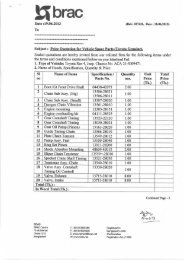
![[re-tender] RFQ for supply of Diesel Generator - Brac](https://img.yumpu.com/44421374/1/186x260/re-tender-rfq-for-supply-of-diesel-generator-brac.jpg?quality=85)
