Clinical Manual for Management of the HIV-Infected ... - myCME.com
Clinical Manual for Management of the HIV-Infected ... - myCME.com
Clinical Manual for Management of the HIV-Infected ... - myCME.com
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
3–28 | <strong>Clinical</strong> <strong>Manual</strong> <strong>for</strong> <strong>Management</strong> <strong>of</strong> <strong>the</strong> <strong>HIV</strong>-<strong>Infected</strong> Adult/2006<br />
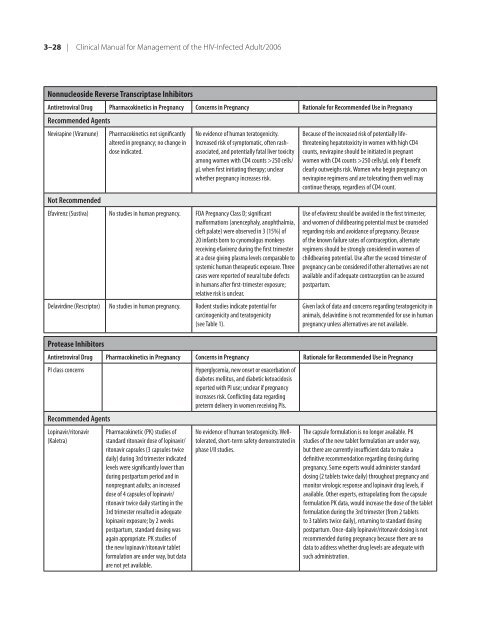
Nonnucleoside Reverse Transcriptase Inhibitors<br />
Antiretroviral Drug Pharmacokinetics in Pregnancy Concerns in Pregnancy Rationale <strong>for</strong> Re<strong>com</strong>mended Use in Pregnancy<br />
Re<strong>com</strong>mended Agents<br />
Nevirapine (Viramune) Pharmacokinetics not significantly<br />
altered in pregnancy; no change in<br />
dose indicated.<br />
Not Re<strong>com</strong>mended<br />
No evidence <strong>of</strong> human teratogenicity.<br />
Increased risk <strong>of</strong> symptomatic, <strong>of</strong>ten rashassociated,<br />
and potentially fatal liver toxicity<br />
among women with CD4 counts >250 cells/<br />
µL when first initiating <strong>the</strong>rapy; unclear<br />
whe<strong>the</strong>r pregnancy increases risk.<br />
Efavirenz (Sustiva) No studies in human pregnancy. FDA Pregnancy Class D; significant<br />
mal<strong>for</strong>mations (anencephaly, anophthalmia,<br />
cleft palate) were observed in 3 (15%) <strong>of</strong><br />
20 infants born to cynomolgus monkeys<br />
receiving efavirenz during <strong>the</strong> first trimester<br />
at a dose giving plasma levels <strong>com</strong>parable to<br />
systemic human <strong>the</strong>rapeutic exposure. Three<br />
cases were reported <strong>of</strong> neural tube defects<br />
in humans after first-trimester exposure;<br />
relative risk is unclear.<br />
Delavirdine (Rescriptor) No studies in human pregnancy. Rodent studies indicate potential <strong>for</strong><br />
carcinogenicity and teratogenicity<br />
(see Table 1).<br />
Protease Inhibitors<br />
Because <strong>of</strong> <strong>the</strong> increased risk <strong>of</strong> potentially lifethreatening<br />
hepatotoxicity in women with high CD4<br />
counts, nevirapine should be initiated in pregnant<br />
women with CD4 counts >250 cells/µL only if benefit<br />
clearly outweighs risk. Women who begin pregnancy on<br />
nevirapine regimens and are tolerating <strong>the</strong>m well may<br />
continue <strong>the</strong>rapy, regardless <strong>of</strong> CD4 count.<br />
Use <strong>of</strong> efavirenz should be avoided in <strong>the</strong> first trimester,<br />
and women <strong>of</strong> childbearing potential must be counseled<br />
regarding risks and avoidance <strong>of</strong> pregnancy. Because<br />
<strong>of</strong> <strong>the</strong> known failure rates <strong>of</strong> contraception, alternate<br />
regimens should be strongly considered in women <strong>of</strong><br />
childbearing potential. Use after <strong>the</strong> second trimester <strong>of</strong><br />
pregnancy can be considered if o<strong>the</strong>r alternatives are not<br />
available and if adequate contraception can be assured<br />
postpartum.<br />
Given lack <strong>of</strong> data and concerns regarding teratogenicity in<br />
animals, delavirdine is not re<strong>com</strong>mended <strong>for</strong> use in human<br />
pregnancy unless alternatives are not available.<br />
Antiretroviral Drug Pharmacokinetics in Pregnancy Concerns in Pregnancy Rationale <strong>for</strong> Re<strong>com</strong>mended Use in Pregnancy<br />
PI class concerns Hyperglycemia, new onset or exacerbation <strong>of</strong><br />
diabetes mellitus, and diabetic ketoacidosis<br />
reported with PI use; unclear if pregnancy<br />
increases risk. Conflicting data regarding<br />
preterm delivery in women receiving PIs.<br />
Re<strong>com</strong>mended Agents<br />
Lopinavir/ritonavir<br />
(Kaletra)<br />
Pharmacokinetic (PK) studies <strong>of</strong><br />
standard ritonavir dose <strong>of</strong> lopinavir/<br />
ritonavir capsules (3 capsules twice<br />
daily) during 3rd trimester indicated<br />
levels were significantly lower than<br />
during postpartum period and in<br />
nonpregnant adults; an increased<br />
dose <strong>of</strong> 4 capsules <strong>of</strong> lopinavir/<br />
ritonavir twice daily starting in <strong>the</strong><br />
3rd trimester resulted in adequate<br />
lopinavir exposure; by 2 weeks<br />
postpartum, standard dosing was<br />
again appropriate. PK studies <strong>of</strong><br />
<strong>the</strong> new lopinavir/ritonavir tablet<br />
<strong>for</strong>mulation are under way, but data<br />
are not yet available.<br />
No evidence <strong>of</strong> human teratogenicity. Welltolerated,<br />
short-term safety demonstrated in<br />
phase I/II studies.<br />
The capsule <strong>for</strong>mulation is no longer available. PK<br />
studies <strong>of</strong> <strong>the</strong> new tablet <strong>for</strong>mulation are under way,<br />
but <strong>the</strong>re are currently insufficient data to make a<br />
definitive re<strong>com</strong>mendation regarding dosing during<br />
pregnancy. Some experts would administer standard<br />
dosing (2 tablets twice daily) throughout pregnancy and<br />
monitor virologic response and lopinavir drug levels, if<br />
available. O<strong>the</strong>r experts, extrapolating from <strong>the</strong> capsule<br />
<strong>for</strong>mulation PK data, would increase <strong>the</strong> dose <strong>of</strong> <strong>the</strong> tablet<br />
<strong>for</strong>mulation during <strong>the</strong> 3rd trimester (from 2 tablets<br />
to 3 tablets twice daily), returning to standard dosing<br />
postpartum. Once-daily lopinavir/ritonavir dosing is not<br />
re<strong>com</strong>mended during pregnancy because <strong>the</strong>re are no<br />
data to address whe<strong>the</strong>r drug levels are adequate with<br />
such administration.