Clinical Manual for Management of the HIV-Infected ... - myCME.com
Clinical Manual for Management of the HIV-Infected ... - myCME.com
Clinical Manual for Management of the HIV-Infected ... - myCME.com
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
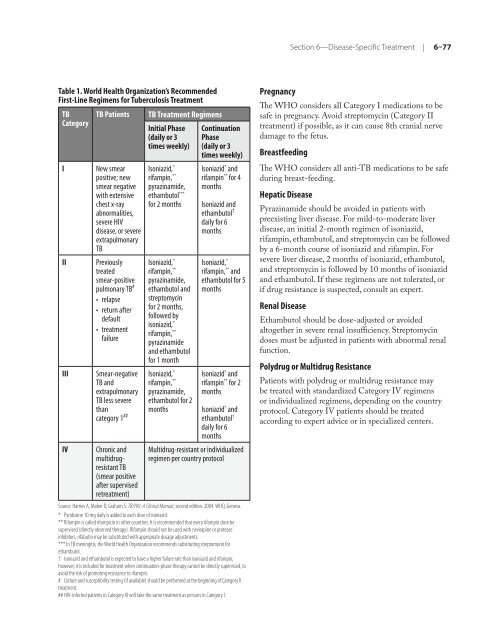
Table 1. World Health Organization’s Re<strong>com</strong>mended<br />
First-Line Regimens <strong>for</strong> Tuberculosis Treatment<br />
TB<br />
Category<br />
TB Patients TB Treatment Regimens<br />
I New smear<br />
positive; new<br />
smear negative<br />
with extensive<br />
chest x-ray<br />
abnormalities,<br />
severe <strong>HIV</strong><br />
disease, or severe<br />
extrapulmonary<br />
TB<br />
II Previously<br />
treated<br />
smear-positive<br />
pulmonary TB #<br />
•<br />
•<br />
•<br />
relapse<br />
return after<br />
default<br />
treatment<br />
failure<br />
III Smear-negative<br />
TB and<br />
extrapulmonary<br />
TB less severe<br />
than<br />
category 1 ##<br />
IV Chronic and<br />
multidrugresistant<br />
TB<br />
(smear positive<br />
after supervised<br />
retreatment)<br />
Initial Phase<br />
(daily or 3<br />
times weekly)<br />
Isoniazid, *<br />
rifampin, **<br />
pyrazinamide,<br />
ethambutol ***<br />
<strong>for</strong> 2 months<br />
Isoniazid, *<br />
rifampin, **<br />
pyrazinamide,<br />
ethambutol and<br />
streptomycin<br />
<strong>for</strong> 2 months,<br />
followed by<br />
isoniazid, *<br />
rifampin, **<br />
pyrazinamide<br />
and ethambutol<br />
<strong>for</strong> 1 month<br />
Isoniazid, *<br />
rifampin, **<br />
pyrazinamide,<br />
ethambutol <strong>for</strong> 2<br />
months<br />
Continuation<br />
Phase<br />
(daily or 3<br />
times weekly)<br />
Isoniazid * and<br />
rifampin ** <strong>for</strong> 4<br />
months<br />
Isoniazid and<br />
ethambutol †<br />
daily <strong>for</strong> 6<br />
months<br />
Isoniazid, *<br />
rifampin, ** and<br />
ethambutol <strong>for</strong> 5<br />
months<br />
Isoniazid * and<br />
rifampin ** <strong>for</strong> 2<br />
months<br />
Isoniazid * and<br />
ethambutol †<br />
daily <strong>for</strong> 6<br />
months<br />
Multidrug-resistant or individualized<br />
regimen per country protocol<br />
Source: Harries A, Maher D, Graham S. TB/<strong>HIV</strong>: A <strong>Clinical</strong> <strong>Manual</strong>, second edition. 2004. WHO, Geneva.<br />
* Pyridoxine 10 mg daily is added to each dose <strong>of</strong> isoniazid.<br />
** Rifampin is called rifampicin in o<strong>the</strong>r countries. It is re<strong>com</strong>mended that every rifampin dose be<br />
supervised (directly observed <strong>the</strong>rapy). Rifampin should not be used with nevirapine or protease<br />
inhibitors; rifabutin may be substituted with appropriate dosage adjustments.<br />
*** In TB meningitis, <strong>the</strong> World Health Organization re<strong>com</strong>mends substituting streptomycin <strong>for</strong><br />
ethambutol.<br />
† Isoniazid and ethambutol is expected to have a higher failure rate than isoniazid and rifampin;<br />
however, it is included <strong>for</strong> treatment when continuation-phase <strong>the</strong>rapy cannot be directly supervised, to<br />
avoid <strong>the</strong> risk <strong>of</strong> promoting resistance to rifampin.<br />
# Culture and susceptibility testing (if available) should be per<strong>for</strong>med at <strong>the</strong> beginning <strong>of</strong> Category II<br />
treatment.<br />
## <strong>HIV</strong>-infected patients in Category III will take <strong>the</strong> same treatment as persons in Category I.<br />
Pregnancy<br />
Section 6—Disease-Specific Treatment | 6–77<br />
The WHO considers all Category I medications to be<br />
safe in pregnancy. Avoid streptomycin (Category II<br />
treatment) if possible, as it can cause 8th cranial nerve<br />
damage to <strong>the</strong> fetus.<br />
Breastfeeding<br />
The WHO considers all anti-TB medications to be safe<br />
during breast-feeding.<br />
Hepatic Disease<br />
Pyrazinamide should be avoided in patients with<br />
preexisting liver disease. For mild-to-moderate liver<br />
disease, an initial 2-month regimen <strong>of</strong> isoniazid,<br />
rifampin, ethambutol, and streptomycin can be followed<br />
by a 6-month course <strong>of</strong> isoniazid and rifampin. For<br />
severe liver disease, 2 months <strong>of</strong> isoniazid, ethambutol,<br />
and streptomycin is followed by 10 months <strong>of</strong> isoniazid<br />
and ethambutol. If <strong>the</strong>se regimens are not tolerated, or<br />
if drug resistance is suspected, consult an expert.<br />
Renal Disease<br />
Ethambutol should be dose-adjusted or avoided<br />
altoge<strong>the</strong>r in severe renal insufficiency. Streptomycin<br />
doses must be adjusted in patients with abnormal renal<br />
function.<br />
Polydrug or Multidrug Resistance<br />
Patients with polydrug or multidrug resistance may<br />
be treated with standardized Category IV regimens<br />
or individualized regimens, depending on <strong>the</strong> country<br />
protocol. Category IV patients should be treated<br />
according to expert advice or in specialized centers.