Clinical Manual for Management of the HIV-Infected ... - myCME.com
Clinical Manual for Management of the HIV-Infected ... - myCME.com
Clinical Manual for Management of the HIV-Infected ... - myCME.com
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
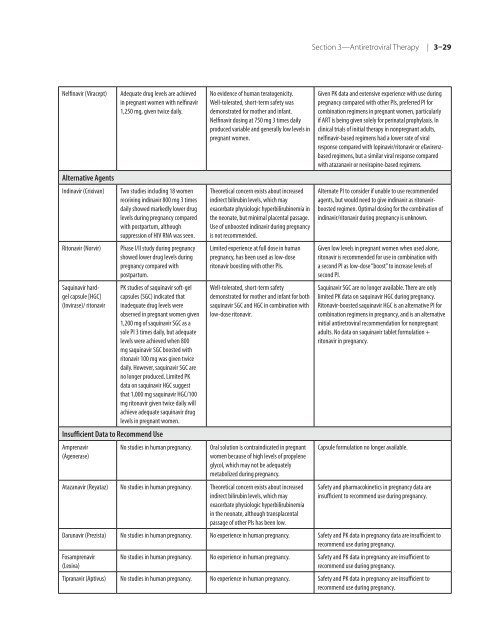
Nelfinavir (Viracept) Adequate drug levels are achieved<br />
in pregnant women with nelfinavir<br />
1,250 mg, given twice daily.<br />
Alternative Agents<br />
Indinavir (Crixivan) Two studies including 18 women<br />
receiving indinavir 800 mg 3 times<br />
daily showed markedly lower drug<br />
levels during pregnancy <strong>com</strong>pared<br />
with postpartum, although<br />
suppression <strong>of</strong> <strong>HIV</strong> RNA was seen.<br />
Ritonavir (Norvir) Phase I/II study during pregnancy<br />
showed lower drug levels during<br />
pregnancy <strong>com</strong>pared with<br />
postpartum.<br />
Saquinavir hardgel<br />
capsule [HGC]<br />
(Invirase)/ ritonavir<br />
PK studies <strong>of</strong> saquinavir s<strong>of</strong>t-gel<br />
capsules (SGC) indicated that<br />
inadequate drug levels were<br />
observed in pregnant women given<br />
1,200 mg <strong>of</strong> saquinavir SGC as a<br />
sole PI 3 times daily, but adequate<br />
levels were achieved when 800<br />
mg saquinavir SGC boosted with<br />
ritonavir 100 mg was given twice<br />
daily. However, saquinavir SGC are<br />
no longer produced. Limited PK<br />
data on saquinavir HGC suggest<br />
that 1,000 mg saquinavir HGC/100<br />
mg ritonavir given twice daily will<br />
achieve adequate saquinavir drug<br />
levels in pregnant women.<br />
Insufficient Data to Re<strong>com</strong>mend Use<br />
Amprenavir<br />
(Agenerase)<br />
No evidence <strong>of</strong> human teratogenicity.<br />
Well-tolerated, short-term safety was<br />
demonstrated <strong>for</strong> mo<strong>the</strong>r and infant.<br />
Nelfinavir dosing at 750 mg 3 times daily<br />
produced variable and generally low levels in<br />
pregnant women.<br />
Theoretical concern exists about increased<br />
indirect bilirubin levels, which may<br />
exacerbate physiologic hyperbilirubinemia in<br />
<strong>the</strong> neonate, but minimal placental passage.<br />
Use <strong>of</strong> unboosted indinavir during pregnancy<br />
is not re<strong>com</strong>mended.<br />
Limited experience at full dose in human<br />
pregnancy, has been used as low-dose<br />
ritonavir boosting with o<strong>the</strong>r PIs.<br />
Well-tolerated, short-term safety<br />
demonstrated <strong>for</strong> mo<strong>the</strong>r and infant <strong>for</strong> both<br />
saquinavir SGC and HGC in <strong>com</strong>bination with<br />
low-dose ritonavir.<br />
No studies in human pregnancy. Oral solution is contraindicated in pregnant<br />
women because <strong>of</strong> high levels <strong>of</strong> propylene<br />
glycol, which may not be adequately<br />
metabolized during pregnancy.<br />
Atazanavir (Reyataz) No studies in human pregnancy. Theoretical concern exists about increased<br />
indirect bilirubin levels, which may<br />
exacerbate physiologic hyperbilirubinemia<br />
in <strong>the</strong> neonate, although transplacental<br />
passage <strong>of</strong> o<strong>the</strong>r PIs has been low.<br />
Section 3—Antiretroviral Therapy | 3–29<br />
Given PK data and extensive experience with use during<br />
pregnancy <strong>com</strong>pared with o<strong>the</strong>r PIs, preferred PI <strong>for</strong><br />
<strong>com</strong>bination regimens in pregnant women, particularly<br />
if ART is being given solely <strong>for</strong> perinatal prophylaxis. In<br />
clinical trials <strong>of</strong> initial <strong>the</strong>rapy in nonpregnant adults,<br />
nelfinavir-based regimens had a lower rate <strong>of</strong> viral<br />
response <strong>com</strong>pared with lopinavir/ritonavir or efavirenzbased<br />
regimens, but a similar viral response <strong>com</strong>pared<br />
with atazanavir or nevirapine-based regimens.<br />
Alternate PI to consider if unable to use re<strong>com</strong>mended<br />
agents, but would need to give indinavir as ritonavirboosted<br />
regimen. Optimal dosing <strong>for</strong> <strong>the</strong> <strong>com</strong>bination <strong>of</strong><br />
indinavir/ritonavir during pregnancy is unknown.<br />
Given low levels in pregnant women when used alone,<br />
ritonavir is re<strong>com</strong>mended <strong>for</strong> use in <strong>com</strong>bination with<br />
a second PI as low-dose “boost” to increase levels <strong>of</strong><br />
second PI.<br />
Saquinavir SGC are no longer available. There are only<br />
limited PK data on saquinavir HGC during pregnancy.<br />
Ritonavir-boosted saquinavir HGC is an alternative PI <strong>for</strong><br />
<strong>com</strong>bination regimens in pregnancy, and is an alternative<br />
initial antiretroviral re<strong>com</strong>mendation <strong>for</strong> nonpregnant<br />
adults. No data on saquinavir tablet <strong>for</strong>mulation +<br />
ritonavir in pregnancy.<br />
Capsule <strong>for</strong>mulation no longer available.<br />
Safety and pharmacokinetics in pregnancy data are<br />
insufficient to re<strong>com</strong>mend use during pregnancy.<br />
Darunavir (Prezista) No studies in human pregnancy. No experience in human pregnancy. Safety and PK data in pregnancy data are insufficient to<br />
re<strong>com</strong>mend use during pregnancy.<br />
Fosamprenavir<br />
(Lexiva)<br />
No studies in human pregnancy. No experience in human pregnancy. Safety and PK data in pregnancy are insufficient to<br />
re<strong>com</strong>mend use during pregnancy.<br />
Tipranavir (Aptivus) No studies in human pregnancy. No experience in human pregnancy. Safety and PK data in pregnancy are insufficient to<br />
re<strong>com</strong>mend use during pregnancy.