Clinical Manual for Management of the HIV-Infected ... - myCME.com
Clinical Manual for Management of the HIV-Infected ... - myCME.com
Clinical Manual for Management of the HIV-Infected ... - myCME.com
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
6–78 | <strong>Clinical</strong> <strong>Manual</strong> <strong>for</strong> <strong>Management</strong> <strong>of</strong> <strong>the</strong> <strong>HIV</strong>-<strong>Infected</strong> Adult/2006<br />
Monitoring <strong>for</strong> Treatment Effectiveness<br />
Patients who are smear positive initially should be<br />
monitored by repeat sputum smears (Table 2). Young<br />
children, smear-negative pulmonary TB patients, and<br />
extrapulmonary TB patients can be followed clinically.<br />
Repeat chest x-ray is not re<strong>com</strong>mended <strong>for</strong> routine<br />
follow-up and is considered a poor use <strong>of</strong> resources.<br />
Chest x-ray should be repeated only <strong>for</strong> a patient with<br />
new or progressive symptoms.<br />
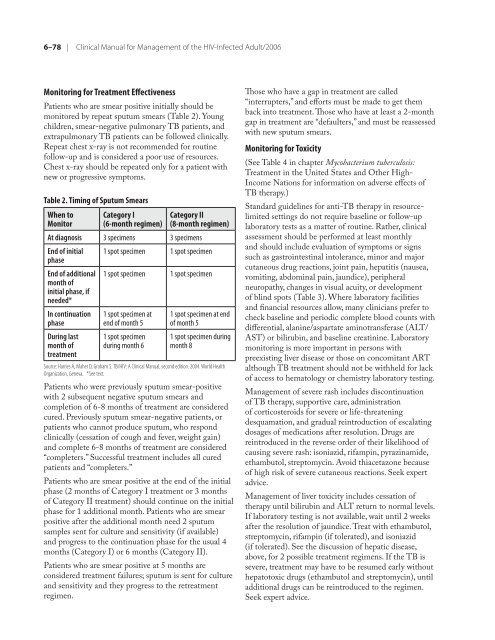
Table 2. Timing <strong>of</strong> Sputum Smears<br />
When to<br />
Monitor<br />
Category I<br />
(6-month regimen)<br />
Category II<br />
(8-month regimen)<br />
At diagnosis 3 specimens 3 specimens<br />
End <strong>of</strong> initial<br />
phase<br />
End <strong>of</strong> additional<br />
month <strong>of</strong><br />
initial phase, if<br />
needed*<br />
In continuation<br />
phase<br />
During last<br />
month <strong>of</strong><br />
treatment<br />
1 spot specimen 1 spot specimen<br />
1 spot specimen 1 spot specimen<br />
1 spot specimen at<br />
end <strong>of</strong> month 5<br />
1 spot specimen<br />
during month 6<br />
1 spot specimen at end<br />
<strong>of</strong> month 5<br />
1 spot specimen during<br />
month 8<br />
Source: Harries A, Maher D, Graham S. TB/<strong>HIV</strong>: A <strong>Clinical</strong> <strong>Manual</strong>, second edition. 2004. World Health<br />
Organization, Geneva. *See text.<br />
Patients who were previously sputum smear-positive<br />
with 2 subsequent negative sputum smears and<br />
<strong>com</strong>pletion <strong>of</strong> 6-8 months <strong>of</strong> treatment are considered<br />
cured. Previously sputum smear-negative patients, or<br />
patients who cannot produce sputum, who respond<br />
clinically (cessation <strong>of</strong> cough and fever, weight gain)<br />
and <strong>com</strong>plete 6-8 months <strong>of</strong> treatment are considered<br />
“<strong>com</strong>pleters.” Successful treatment includes all cured<br />
patients and “<strong>com</strong>pleters.”<br />
Patients who are smear positive at <strong>the</strong> end <strong>of</strong> <strong>the</strong> initial<br />
phase (2 months <strong>of</strong> Category I treatment or 3 months<br />
<strong>of</strong> Category II treatment) should continue on <strong>the</strong> initial<br />
phase <strong>for</strong> 1 additional month. Patients who are smear<br />
positive after <strong>the</strong> additional month need 2 sputum<br />
samples sent <strong>for</strong> culture and sensitivity (if available)<br />
and progress to <strong>the</strong> continuation phase <strong>for</strong> <strong>the</strong> usual 4<br />
months (Category I) or 6 months (Category II).<br />
Patients who are smear positive at 5 months are<br />
considered treatment failures; sputum is sent <strong>for</strong> culture<br />
and sensitivity and <strong>the</strong>y progress to <strong>the</strong> retreatment<br />
regimen.<br />
Those who have a gap in treatment are called<br />
“interrupters,” and ef<strong>for</strong>ts must be made to get <strong>the</strong>m<br />
back into treatment. Those who have at least a 2-month<br />
gap in treatment are “defaulters,” and must be reassessed<br />
with new sputum smears.<br />
Monitoring <strong>for</strong> Toxicity<br />
(See Table 4 in chapter Mycobacterium tuberculosis:<br />
Treatment in <strong>the</strong> United States and O<strong>the</strong>r High-<br />
In<strong>com</strong>e Nations <strong>for</strong> in<strong>for</strong>mation on adverse effects <strong>of</strong><br />
TB <strong>the</strong>rapy.)<br />
Standard guidelines <strong>for</strong> anti-TB <strong>the</strong>rapy in resourcelimited<br />
settings do not require baseline or follow-up<br />
laboratory tests as a matter <strong>of</strong> routine. Ra<strong>the</strong>r, clinical<br />
assessment should be per<strong>for</strong>med at least monthly<br />
and should include evaluation <strong>of</strong> symptoms or signs<br />
such as gastrointestinal intolerance, minor and major<br />
cutaneous drug reactions, joint pain, hepatitis (nausea,<br />
vomiting, abdominal pain, jaundice), peripheral<br />
neuropathy, changes in visual acuity, or development<br />
<strong>of</strong> blind spots (Table 3). Where laboratory facilities<br />
and financial resources allow, many clinicians prefer to<br />
check baseline and periodic <strong>com</strong>plete blood counts with<br />
differential, alanine/aspartate aminotransferase (ALT/<br />
AST) or bilirubin, and baseline creatinine. Laboratory<br />
monitoring is more important in persons with<br />
preexisting liver disease or those on con<strong>com</strong>itant ART<br />
although TB treatment should not be withheld <strong>for</strong> lack<br />
<strong>of</strong> access to hematology or chemistry laboratory testing.<br />
<strong>Management</strong> <strong>of</strong> severe rash includes discontinuation<br />
<strong>of</strong> TB <strong>the</strong>rapy, supportive care, administration<br />
<strong>of</strong> corticosteroids <strong>for</strong> severe or life-threatening<br />
desquamation, and gradual reintroduction <strong>of</strong> escalating<br />
dosages <strong>of</strong> medications after resolution. Drugs are<br />
reintroduced in <strong>the</strong> reverse order <strong>of</strong> <strong>the</strong>ir likelihood <strong>of</strong><br />
causing severe rash: isoniazid, rifampin, pyrazinamide,<br />
ethambutol, streptomycin. Avoid thiacetazone because<br />
<strong>of</strong> high risk <strong>of</strong> severe cutaneous reactions. Seek expert<br />
advice.<br />
<strong>Management</strong> <strong>of</strong> liver toxicity includes cessation <strong>of</strong><br />
<strong>the</strong>rapy until bilirubin and ALT return to normal levels.<br />
If laboratory testing is not available, wait until 2 weeks<br />
after <strong>the</strong> resolution <strong>of</strong> jaundice. Treat with ethambutol,<br />
streptomycin, rifampin (if tolerated), and isoniazid<br />
(if tolerated). See <strong>the</strong> discussion <strong>of</strong> hepatic disease,<br />
above, <strong>for</strong> 2 possible treatment regimens. If <strong>the</strong> TB is<br />
severe, treatment may have to be resumed early without<br />
hepatotoxic drugs (ethambutol and streptomycin), until<br />
additional drugs can be reintroduced to <strong>the</strong> regimen.<br />
Seek expert advice.