Acute Leukemias - Republican Scientific Medical Library
Acute Leukemias - Republican Scientific Medical Library
Acute Leukemias - Republican Scientific Medical Library
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
138 Chapter 10 · Recent Clinical Trials in <strong>Acute</strong> Lymphoblastic Leukemia by the Cancer and Leukemia Group B<br />
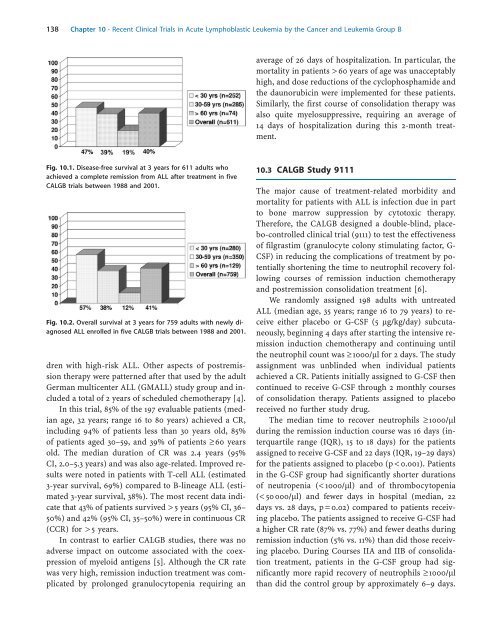
Fig. 10.1. Disease-free survival at 3 years for 611 adults who<br />
achieved a complete remission from ALL after treatment in five<br />
CALGB trials between 1988 and 2001.<br />
Fig. 10.2. Overall survival at 3 years for 759 adults with newly diagnosed<br />
ALL enrolled in five CALGB trials between 1988 and 2001.<br />
dren with high-risk ALL. Other aspects of postremission<br />
therapy were patterned after that used by the adult<br />
German multicenter ALL (GMALL) study group and included<br />
a total of 2 years of scheduled chemotherapy [4].<br />
In this trial, 85% of the 197 evaluable patients (median<br />
age, 32 years; range 16 to 80 years) achieved a CR,<br />
including 94% of patients less than 30 years old, 85%<br />
of patients aged 30–59, and 39% of patients ³60 years<br />
old. The median duration of CR was 2.4 years (95%<br />
CI, 2.0–5.3 years) and was also age-related. Improved results<br />
were noted in patients with T-cell ALL (estimated<br />
3-year survival, 69%) compared to B-lineage ALL (estimated<br />
3-year survival, 38%). The most recent data indicate<br />
that 43% of patients survived > 5 years (95% CI, 36–<br />
50%) and 42% (95% CI, 35–50%) were in continuous CR<br />
(CCR) for >5 years.<br />
In contrast to earlier CALGB studies, there was no<br />
adverse impact on outcome associated with the coexpression<br />
of myeloid antigens [5]. Although the CR rate<br />
was very high, remission induction treatment was complicated<br />
by prolonged granulocytopenia requiring an<br />
average of 26 days of hospitalization. In particular, the<br />
mortality in patients >60 years of age was unacceptably<br />
high, and dose reductions of the cyclophosphamide and<br />
the daunorubicin were implemented for these patients.<br />
Similarly, the first course of consolidation therapy was<br />
also quite myelosuppressive, requiring an average of<br />
14 days of hospitalization during this 2-month treatment.<br />
10.3 CALGB Study 9111<br />
The major cause of treatment-related morbidity and<br />
mortality for patients with ALL is infection due in part<br />
to bone marrow suppression by cytotoxic therapy.<br />
Therefore, the CALGB designed a double-blind, placebo-controlled<br />
clinical trial (9111) to test the effectiveness<br />
of filgrastim (granulocyte colony stimulating factor, G-<br />
CSF) in reducing the complications of treatment by potentially<br />
shortening the time to neutrophil recovery following<br />
courses of remission induction chemotherapy<br />
and postremission consolidation treatment [6].<br />
We randomly assigned 198 adults with untreated<br />
ALL (median age, 35 years; range 16 to 79 years) to receive<br />
either placebo or G-CSF (5 lg/kg/day) subcutaneously,<br />
beginning 4 days after starting the intensive remission<br />
induction chemotherapy and continuing until<br />
the neutrophil count was ³1000/ll for 2 days. The study<br />
assignment was unblinded when individual patients<br />
achieved a CR. Patients initially assigned to G-CSF then<br />
continued to receive G-CSF through 2 monthly courses<br />
of consolidation therapy. Patients assigned to placebo<br />
received no further study drug.<br />
The median time to recover neutrophils ³1000/ll<br />
during the remission induction course was 16 days (interquartile<br />
range (IQR), 15 to 18 days) for the patients<br />
assigned to receive G-CSF and 22 days (IQR, 19–29 days)<br />
for the patients assigned to placebo (p < 0.001). Patients<br />
in the G-CSF group had significantly shorter durations<br />
of neutropenia (< 1000/ll) and of thrombocytopenia<br />
(< 50000/ll) and fewer days in hospital (median, 22<br />
days vs. 28 days, p=0.02) compared to patients receiving<br />
placebo. The patients assigned to receive G-CSF had<br />
a higher CR rate (87% vs. 77%) and fewer deaths during<br />
remission induction (5% vs. 11%) than did those receiving<br />
placebo. During Courses IIA and IIB of consolidation<br />
treatment, patients in the G-CSF group had significantly<br />
more rapid recovery of neutrophils ³1000/ll<br />
than did the control group by approximately 6–9 days.