Volume 6, Spring 2008 - Saddleback College
Volume 6, Spring 2008 - Saddleback College
Volume 6, Spring 2008 - Saddleback College
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Fall 2007 Biology 3A Abstracts<br />
understanding of using silver nitrate (AgNO 3 ) to<br />
inhibit the growth of the bacteria Escherichia coli.<br />
An article published by Nataro and Kaper (1998) in<br />
the Clinical Microbiology Reviews, Escherichia coli<br />
is the predominant nonpathogenic facultative flora of<br />
the human intestine. Some E. coli strains, however,<br />
have developed the ability to cause disease of the<br />
gastrointestinal, urinary, or central nervous system in<br />
even the most robust human hosts. This makes quite a<br />
challenge in the inhibiting treatment of E. coli.<br />
According to Feng and Wu (2000) on the<br />
antibacterial effect of silver ions on E. coli and<br />
Staphylococcus aureus after the use of silver ions<br />
there were morphological changes in both bacteria.<br />
Silver is an agent known to have antibacterial<br />
properties and its inhibiting effect on E. coli would<br />
be a very cost effective way to treat outbreaks of this<br />
bacteria. Investigators Sondi and Sondi (2004) at the<br />
Center for Marine and Environmental Research<br />
found that nanosized silver particles damaged E. coli<br />
cells, showing formation of “pits” in the cell wall of<br />
the bacteria, while the silver nanoparticles were<br />
found to accumulate in the bacterial membrane. A<br />
membrane with such morphology exhibits a<br />
significant increase in permeability, resulting in the<br />
death of the cell. Schreurs and Rosenberg (1982)<br />
concluded that silver ions inhibit the respiratory chain<br />
of E. coli possibly at two sites of different<br />
sensitivities, and were also reported, in the journal of<br />
bacteriology, to exert an uncoupler-like action. It<br />
seems that silver will affect the E. coli in some way<br />
most often damaging the replication process and<br />
contributing to the death of the cell. Rosenkranz and<br />
Carr found that silver sulfadiazine blocked<br />
macromolecular synthesis in treated bacteria. Silver<br />
has been a known antibacterial agent against gram<br />
negative bacteria and could also have an effect on the<br />
gram positive bacteria E. coli. Gram positive bacteria<br />
is very resistant to antibiotic treatment. According to<br />
Jack and Tagg (1995) the journal of microbiology<br />
and molecular biology reviews published an article<br />
on the bacteriocins of gram positive bacteria and<br />
stated that the antibacterial action against a sensitive<br />
cell of a gram-positive strain is produced principally<br />
by destabilization of membrane functions. Silver<br />
nitrate is a chemical agent with properties that may<br />
demobilize the growth of E. coli. The hypothesis for<br />
this experiment is that the agar with the highest<br />
concentration of Silver Nitrate will have the greatest<br />
effects on inhibiting the growth of Escherichia coli.<br />
We anticipate the inability for the E. coli eukaryotic<br />
cells to replicate with the treatment of silver nitrate.<br />
department were used to prepare an agar solution<br />
within 500mL of water to determine the inhibition<br />
growth of Escherichia coli. The agar solution was<br />
introduced to fifteen petri dishes all correctly labeled<br />
to the amount of silver nitrate they were introduced to<br />
(0%, 0.5%, and 1.0%). After the agar solution<br />
hardened, 0.5mL of Escherichia coli were pipetted<br />
with a P1000 micro pipette onto each plate and<br />
spread with a sterile glass rod to form a lawn. The<br />
glass rod was dipped into ethanol, flamed, and cooled<br />
in between each plate. Following the incorporation of<br />
E. coli silver nitrate was placed in both the 0.5% and<br />
1.0% labeled plates. The 0% sample was used as a<br />
control group and left without silver nitrate.<br />
A 1.0% silver nitrate solution was obtained<br />
and used to make a 0.5% silver nitrate solution by<br />
diluting 1.0mL of it into 1.0mL of water. Chads were<br />
used to incorporate the corresponding silver nitrate<br />
solution into the correctly labeled petri dish. There<br />
were a total of four chads placed across from each<br />
other into each dish. The same procedure was<br />
followed for the remaining concentrations. Before<br />
placing a chad in each plate forceps were sterilized<br />
by dipping them into ethanol, placing them over a<br />
flame, and letting them cooled in between each plate.<br />
This process was strictly followed in order to avoid<br />
any possible contamination that could interfere with<br />
the case being studied. All petri dishes were placed<br />
into a 37°C incubator and results were interpreted<br />
within a 48 hour period. After each 48 hour period<br />
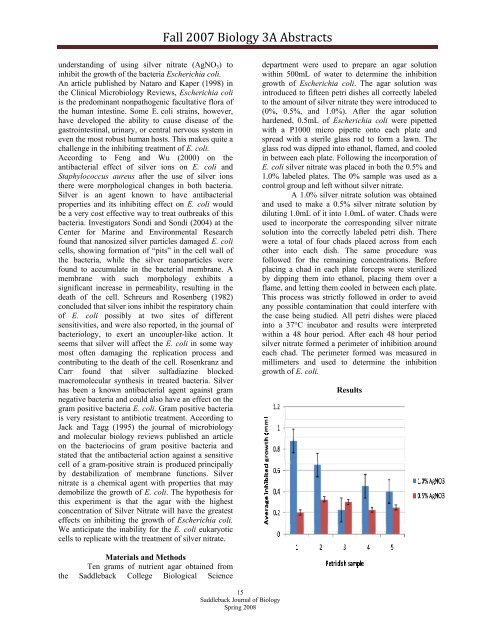
silver nitrate formed a perimeter of inhibition around<br />
each chad. The perimeter formed was measured in<br />
millimeters and used to determine the inhibition<br />
growth of E. coli.<br />
Results<br />
Materials and Methods<br />
Ten grams of nutrient agar obtained from<br />
the <strong>Saddleback</strong> <strong>College</strong> Biological Science<br />
15<br />
<strong>Saddleback</strong> Journal of Biology<br />
<strong>Spring</strong> <strong>2008</strong>