Volume 6, Spring 2008 - Saddleback College
Volume 6, Spring 2008 - Saddleback College
Volume 6, Spring 2008 - Saddleback College
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Fall 2007 Biology 3A Abstracts<br />
turned to a bluish clear color with blue color<br />
precipitate floating in the water.<br />
Conc of N (ppm)<br />
1.8<br />
1.6<br />
1.4<br />
1.2<br />
1<br />
0.8<br />
0.6<br />
0.4<br />
0.2<br />
0<br />
low<br />
high<br />
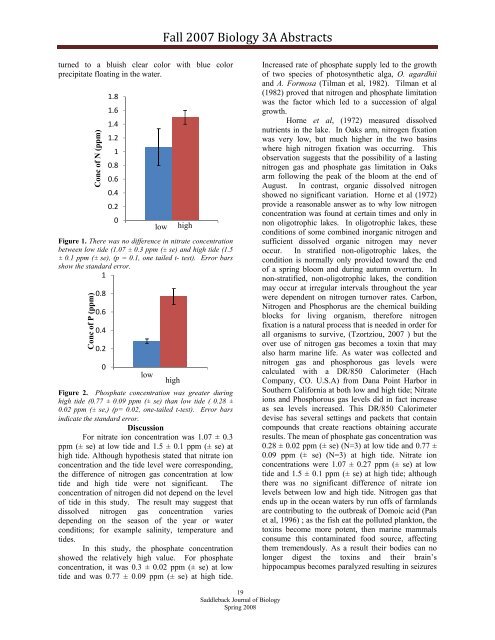
Figure 1. There was no difference in nitrate concentration<br />
between low tide (1.07 ± 0.3 ppm (± se) and high tide (1.5<br />
± 0.1 ppm (± se), (p = 0.1, one tailed t- test). Error bars<br />
show the standard error.<br />
1<br />
Conc of P (ppm)<br />
0.8<br />
0.6<br />
0.4<br />
0.2<br />
0<br />
low<br />
high<br />
Figure 2. Phosphate concentration was greater during<br />
high tide (0.77 ± 0.09 ppm (± se) than low tide ( 0.28 ±<br />
0.02 ppm (± se,) (p= 0.02, one-tailed t-test). Error bars<br />
indicate the standard error.<br />
Discussion<br />
For nitrate ion concentration was 1.07 ± 0.3<br />
ppm (± se) at low tide and 1.5 ± 0.1 ppm (± se) at<br />
high tide. Although hypothesis stated that nitrate ion<br />
concentration and the tide level were corresponding,<br />
the difference of nitrogen gas concentration at low<br />
tide and high tide were not significant. The<br />
concentration of nitrogen did not depend on the level<br />
of tide in this study. The result may suggest that<br />
dissolved nitrogen gas concentration varies<br />
depending on the season of the year or water<br />
conditions; for example salinity, temperature and<br />
tides.<br />
In this study, the phosphate concentration<br />
showed the relatively high value. For phosphate<br />
concentration, it was 0.3 ± 0.02 ppm (± se) at low<br />
tide and was 0.77 ± 0.09 ppm (± se) at high tide.<br />
Increased rate of phosphate supply led to the growth<br />
of two species of photosynthetic alga, O. agardhii<br />
and A. Formosa (Tilman et al, 1982). Tilman et al<br />
(1982) proved that nitrogen and phosphate limitation<br />
was the factor which led to a succession of algal<br />
growth.<br />
Horne et al, (1972) measured dissolved<br />
nutrients in the lake. In Oaks arm, nitrogen fixation<br />
was very low, but much higher in the two basins<br />
where high nitrogen fixation was occurring. This<br />
observation suggests that the possibility of a lasting<br />
nitrogen gas and phosphate gas limitation in Oaks<br />
arm following the peak of the bloom at the end of<br />
August. In contrast, organic dissolved nitrogen<br />
showed no significant variation. Horne et al (1972)<br />
provide a reasonable answer as to why low nitrogen<br />
concentration was found at certain times and only in<br />
non oligotrophic lakes. In oligotrophic lakes, these<br />
conditions of some combined inorganic nitrogen and<br />
sufficient dissolved organic nitrogen may never<br />
occur. In stratified non-oligotrophic lakes, the<br />
condition is normally only provided toward the end<br />
of a spring bloom and during autumn overturn. In<br />
non-stratified, non-oligotrophic lakes, the condition<br />
may occur at irregular intervals throughout the year<br />
were dependent on nitrogen turnover rates. Carbon,<br />
Nitrogen and Phosphorus are the chemical building<br />
blocks for living organism, therefore nitrogen<br />
fixation is a natural process that is needed in order for<br />
all organisms to survive, (Tzortziou, 2007 ) but the<br />
over use of nitrogen gas becomes a toxin that may<br />
also harm marine life. As water was collected and<br />
nitrogen gas and phosphorous gas levels were<br />
calculated with a DR/850 Calorimeter (Hach<br />
Company, CO. U.S.A) from Dana Point Harbor in<br />
Southern California at both low and high tide; Nitrate<br />
ions and Phosphorous gas levels did in fact increase<br />
as sea levels increased. This DR/850 Calorimeter<br />
devise has several settings and packets that contain<br />
compounds that create reactions obtaining accurate<br />
results. The mean of phosphate gas concentration was<br />
0.28 ± 0.02 ppm (± se) (N=3) at low tide and 0.77 ±<br />
0.09 ppm (± se) (N=3) at high tide. Nitrate ion<br />
concentrations were 1.07 ± 0.27 ppm (± se) at low<br />
tide and 1.5 ± 0.1 ppm (± se) at high tide; although<br />
there was no significant difference of nitrate ion<br />
levels between low and high tide. Nitrogen gas that<br />
ends up in the ocean waters by run offs of farmlands<br />
are contributing to the outbreak of Domoic acid (Pan<br />
et al, 1996) ; as the fish eat the polluted plankton, the<br />
toxins become more potent, then marine mammals<br />
consume this contaminated food source, affecting<br />
them tremendously. As a result their bodies can no<br />
longer digest the toxins and their brain’s<br />
hippocampus becomes paralyzed resulting in seizures<br />
19<br />
<strong>Saddleback</strong> Journal of Biology<br />
<strong>Spring</strong> <strong>2008</strong>