Volume 6, Spring 2008 - Saddleback College
Volume 6, Spring 2008 - Saddleback College
Volume 6, Spring 2008 - Saddleback College
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Fall 2007 Biology 3A Abstracts<br />
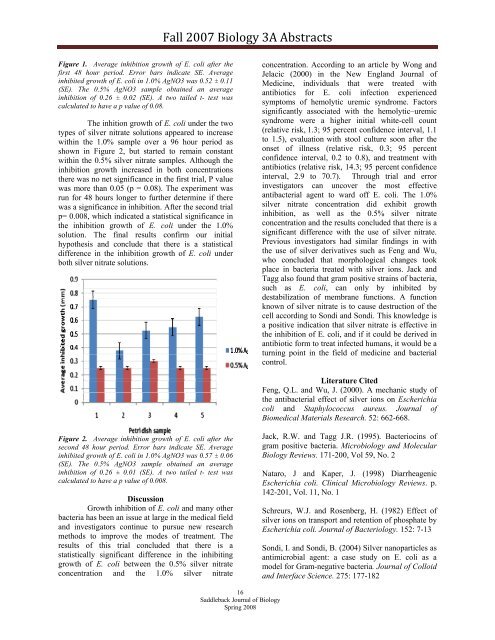
Figure 1. Average inhibition growth of E. coli after the<br />
first 48 hour period. Error bars indicate SE. Average<br />
inhibited growth of E. coli in 1.0% AgNO3 was 0.52 ± 0.11<br />
(SE). The 0.5% AgNO3 sample obtained an average<br />
inhibition of 0.26 ± 0.02 (SE). A two tailed t- test was<br />
calculated to have a p value of 0.08.<br />
The inhition growth of E. coli under the two<br />
types of silver nitrate solutions appeared to increase<br />
within the 1.0% sample over a 96 hour period as<br />
shown in Figure 2, but started to remain constant<br />
within the 0.5% silver nitrate samples. Although the<br />
inhibition growth increased in both concentrations<br />
there was no net significance in the first trial, P value<br />
was more than 0.05 (p = 0.08). The experiment was<br />
run for 48 hours longer to further determine if there<br />
was a significance in inhibition. After the second trial<br />
p= 0.008, which indicated a statistical significance in<br />
the inhibition growth of E. coli under the 1.0%<br />
solution. The final results confirm our initial<br />
hypothesis and conclude that there is a statistical<br />
difference in the inhibition growth of E. coli under<br />
both silver nitrate solutions.<br />
concentration. According to an article by Wong and<br />
Jelacic (2000) in the New England Journal of<br />
Medicine, individuals that were treated with<br />
antibiotics for E. coli infection experienced<br />
symptoms of hemolytic uremic syndrome. Factors<br />
significantly associated with the hemolytic–uremic<br />
syndrome were a higher initial white-cell count<br />
(relative risk, 1.3; 95 percent confidence interval, 1.1<br />
to 1.5), evaluation with stool culture soon after the<br />
onset of illness (relative risk, 0.3; 95 percent<br />
confidence interval, 0.2 to 0.8), and treatment with<br />
antibiotics (relative risk, 14.3; 95 percent confidence<br />
interval, 2.9 to 70.7). Through trial and error<br />
investigators can uncover the most effective<br />
antibacterial agent to ward off E. coli. The 1.0%<br />
silver nitrate concentration did exhibit growth<br />
inhibition, as well as the 0.5% silver nitrate<br />
concentration and the results concluded that there is a<br />
significant difference with the use of silver nitrate.<br />
Previous investigators had similar findings in with<br />
the use of silver derivatives such as Feng and Wu,<br />
who concluded that morphological changes took<br />
place in bacteria treated with silver ions. Jack and<br />
Tagg also found that gram positive strains of bacteria,<br />
such as E. coli, can only by inhibited by<br />
destabilization of membrane functions. A function<br />
known of silver nitrate is to cause destruction of the<br />
cell according to Sondi and Sondi. This knowledge is<br />
a positive indication that silver nitrate is effective in<br />
the inhibition of E. coli, and if it could be derived in<br />
antibiotic form to treat infected humans, it would be a<br />
turning point in the field of medicine and bacterial<br />
control.<br />
Literature Cited<br />
Feng, Q.L. and Wu, J. (2000). A mechanic study of<br />
the antibacterial effect of silver ions on Escherichia<br />
coli and Staphylococcus aureus. Journal of<br />
Biomedical Materials Research. 52: 662-668.<br />
Figure 2. Average inhibition growth of E. coli after the<br />
second 48 hour period. Error bars indicate SE. Average<br />
inhibited growth of E. coli in 1.0% AgNO3 was 0.57 ± 0.06<br />
(SE). The 0.5% AgNO3 sample obtained an average<br />
inhibition of 0.26 ± 0.01 (SE). A two tailed t- test was<br />
calculated to have a p value of 0.008.<br />
Discussion<br />
Growth inhibition of E. coli and many other<br />
bacteria has been an issue at large in the medical field<br />
and investigators continue to pursue new research<br />
methods to improve the modes of treatment. The<br />
results of this trial concluded that there is a<br />
statistically significant difference in the inhibiting<br />
growth of E. coli between the 0.5% silver nitrate<br />
concentration and the 1.0% silver nitrate<br />
Jack, R.W. and Tagg J.R. (1995). Bacteriocins of<br />
gram positive bacteria. Microbiology and Molecular<br />
Biology Reviews. 171-200, Vol 59, No. 2<br />
Nataro, J and Kaper, J. (1998) Diarrheagenic<br />
Escherichia coli. Clinical Microbiology Reviews. p.<br />
142-201, Vol. 11, No. 1<br />
Schreurs, W.J. and Rosenberg, H. (1982) Effect of<br />
silver ions on transport and retention of phosphate by<br />
Escherichia coli. Journal of Bacteriology. 152: 7-13<br />
Sondi, I. and Sondi, B. (2004) Silver nanoparticles as<br />
antimicrobial agent: a case study on E. coli as a<br />
model for Gram-negative bacteria. Journal of Colloid<br />
and Interface Science. 275: 177-182<br />
16<br />
<strong>Saddleback</strong> Journal of Biology<br />
<strong>Spring</strong> <strong>2008</strong>