Stratos®Pro A4... PH User Manual
Stratos®Pro A4... PH User Manual
Stratos®Pro A4... PH User Manual
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
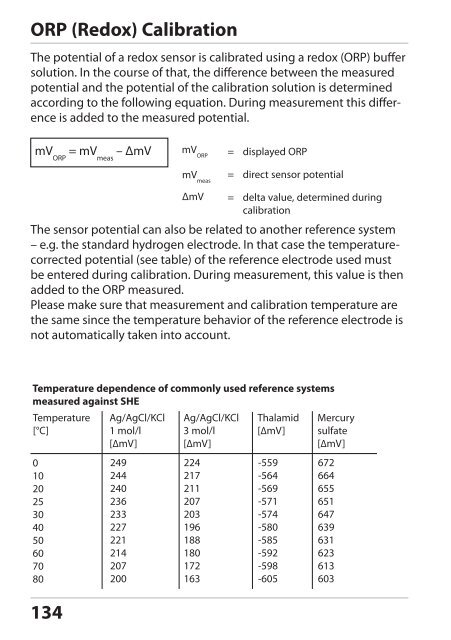
ORP (Redox) Calibration<br />
The potential of a redox sensor is calibrated using a redox (ORP) buffer<br />
solution. In the course of that, the difference between the measured<br />
potential and the potential of the calibration solution is determined<br />
according to the following equation. During measurement this difference<br />
is added to the measured potential.<br />
mV ORP = mV meas – ΔmV<br />
134<br />
mV ORP<br />
mV meas<br />
= displayed ORP<br />
= direct sensor potential<br />
ΔmV = delta value, determined during<br />
calibration<br />
The sensor potential can also be related to another reference system<br />
– e.g. the standard hydrogen electrode. In that case the temperaturecorrected<br />
potential (see table) of the reference electrode used must<br />
be entered during calibration. During measurement, this value is then<br />
added to the ORP measured.<br />
Please make sure that measurement and calibration temperature are<br />
the same since the temperature behavior of the reference electrode is<br />
not automatically taken into account.<br />
Temperature dependence of commonly used reference systems<br />
measured against SHE<br />
Temperature<br />
[°C]<br />
0<br />
10<br />
20<br />
25<br />
30<br />
40<br />
50<br />
60<br />
70<br />
80<br />
Ag/AgCl/KCl<br />
1 mol/l<br />
[∆mV]<br />
249<br />
244<br />
240<br />
236<br />
233<br />
227<br />
221<br />
214<br />
207<br />
200<br />
Ag/AgCl/KCl<br />
3 mol/l<br />
[∆mV]<br />
224<br />
217<br />
211<br />
207<br />
203<br />
196<br />
188<br />
180<br />
172<br />
163<br />
Thalamid<br />
[∆mV]<br />
-559<br />
-564<br />
-569<br />
-571<br />
-574<br />
-580<br />
-585<br />
-592<br />
-598<br />
-605<br />
Mercury<br />
sulfate<br />
[∆mV]<br />
672<br />
664<br />
655<br />
651<br />
647<br />
639<br />
631<br />
623<br />
613<br />
603