12th Congress of the European Hematology ... - Haematologica
12th Congress of the European Hematology ... - Haematologica
12th Congress of the European Hematology ... - Haematologica
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
12 th <strong>Congress</strong> <strong>of</strong> <strong>the</strong> <strong>European</strong> <strong>Hematology</strong> Association<br />
GvHD - GvL - graft rejection<br />
0578<br />
LUNG TRANSPLANTATION AFTER ALLOGENEIC HAEMATOPOIETIC STEM CELL<br />
TRANSPLANTATION<br />
A. Dodds, 1 V. Potter, 1 K. Bradstock, 2 T. O'Brien, 3 A. Glanville1 1 St Vincents Hospital, SYDNEY; 2 Westmead Hospital, SYDNEY, Australia;<br />
3 Sydney Children's Hospital, SYDNEY, Australia<br />
Background and Aim. Pulmonary complications cause significant morbidity<br />
and mortality after haematopoietic allogeneic stem cell transplant<br />
(HSCT). We describe our experience with bilateral lung transplantation<br />
as a <strong>the</strong>rapeutic option in HSCT patients with end-stage lung disease.<br />
Methods. Cases were identified via search <strong>of</strong> database information from<br />
St Vincent’s Hospital and personal contact with lung transplant units in<br />
Australia. Information from medical records <strong>of</strong> identified cases was<br />
analysed. Pathology specimens <strong>of</strong> <strong>the</strong> end stage lung were reviewed.<br />
Results. Five cases have been identified. All received HLA matched allogeneic<br />
transplants from related donors (four sibling, one maternal) for<br />
haematological disease (three CML, one AML, one aplastic anaemia).<br />
Conditioning was with Cy/Bu in four patients and Cy/TBI in one. Graft<br />
versus Host Disease (GVHD) prophylaxis was with CsA/MTX. Acute<br />
GVHD (grades II-III) occurred in two patients. Chronic GVHD o<strong>the</strong>r<br />
than lung occurred in three. Pathology review confirmed <strong>the</strong> diagnosis<br />
<strong>of</strong> bronchiolitis obliterans (BO) in three patients, interstitial fibrosis in a<br />
fourth, and a mixed process in <strong>the</strong> fifth. Time from HSCT to lung transplant<br />
ranged from 23 to 125 months. Four <strong>of</strong> five patients are alive after<br />
lung transplant with no evidence <strong>of</strong> relapse <strong>of</strong> haematological malignancy<br />
(range +8 to+74 months). One patient died three years after lung<br />
transplant from Post-Transplant Lymphoproliferative Disorder. The<br />
remaining patients maintain normal pulmonary function. Morbidity in<br />
<strong>the</strong> living patients includes gastro-oesopharangeal reflux disease, osteoporosis,<br />
hypertension, mild renal impairment, and opportunistic infections<br />
(CMV, aspergillus colonization, MAC reactivation, herpes zoster<br />
and simplex). Marrow function is normal and performance status ECOG<br />
0-1 in <strong>the</strong> surviving patients. Conclusions. Lung transplantation is a viable<br />
<strong>the</strong>rapeutic option for patients with prior HSCT and end-stage lung disease.<br />
Interesting questions requiring fur<strong>the</strong>r research include those related<br />
to causative processes, HLA matching, stem cell dose, prevention<br />
and <strong>the</strong>rapy <strong>of</strong> end stage lung disease. Ongoing immunosuppression<br />
does not appear to affect recipient bone marrow function or contribute<br />
to relapse.<br />
0579<br />
THE GRAFT CONTENT OF DONOR T-CELLS EXPRESSING γ/δ TCR+ AND CD4+FOXP3+<br />
PREDICTS THE RISK OF ACUTE GRAFT VERSUS HOST DISEASE AFTER TRANSPLANTATION<br />
OF ALLOGENEIC PERIPHERAL BLOOD STEM CELLS FROM<br />
UNRELATED DONORS<br />
U. Platzbecker, C. Pabst, G. Ehninger, M. Bornhauser<br />
Universitätsklinikum Carl Gustav Carus, DRESDEN, Germany<br />
Purpose. Recently, high numbers <strong>of</strong> regulatory T-cells within <strong>the</strong> stem<br />
cell graft were described to be associated with less graft versus host disease<br />
(GVHD) after related peripheral blood stem cell transplantation<br />
(PBSCT). Studies in mice also suggest a distinct role <strong>of</strong> γ/δ TCR+ T-cells<br />
in mediating GVHD. Therefore, <strong>the</strong> aim <strong>of</strong> this study was to define <strong>the</strong><br />
yet unknown role <strong>of</strong> regulatory and gamma/delta TCR+ T-cells in<br />
human PBSCT from unrelated donors. Experimental design. The frequency<br />
<strong>of</strong> both T-cell subsets within <strong>the</strong> graft was analyzed in 63 patients<br />
receiving unrelated allogeneic PBSCT. The respective amounts were<br />
quantified by flowcytometry and PCR and fur<strong>the</strong>r correlated with clinical<br />
outcome. Results. The grafts contained a median <strong>of</strong> 11.2×106 /kg<br />
CD4 + foxp3 + and 9.8 ×106 /kg γ/delta TCR + T-cells, respectively. Patients<br />
receiving more CD4 + foxp3 + cells had a lower cumulative incidence <strong>of</strong><br />
acute GVHD II-IV (44% vs. 65%, p=0.03). Interestingly, in patients who<br />
received higher concentrations <strong>of</strong> donor γ/delta TCR + T-cells acute<br />
GVHD II-IV was more frequent (66% vs. 40%, p=0.02). In multivariate<br />
analysis only <strong>the</strong> graft concentration <strong>of</strong> γ/δ TCR + T-cells (p=0.002) and<br />
a positive CMV-status <strong>of</strong> <strong>the</strong> recipient (p=0.03) were significantly associated<br />
with <strong>the</strong> occurrence <strong>of</strong> acute GVHD II-IV. Conclusions. Graft composition<br />
<strong>of</strong> T-cell subsets seems to affect <strong>the</strong> outcome <strong>of</strong> patients receiving<br />
allogeneic PBSCT from unrelated donors. Therefore, selective manipulation<br />
or add-back <strong>of</strong> particular subsets might be a promising strategy<br />
to reduce <strong>the</strong> incidence <strong>of</strong> GVHD.<br />
216 | haematologica/<strong>the</strong> hematology journal | 2007; 92(s1)<br />
0580<br />
TREATMENT OF STEROID REFRACTORY GRAFT VS. HOST DISEASE BY INTRA-ARTERIAL<br />
INFUSION<br />
M.Y. Shapira, A.I. Bloom, R. Or, I.B. Resnick, M. Aker, M. Bitan,<br />
S. Slavin, A. Verstandig<br />
Hadassah Hebrew University Medical Cen, JERUSALEM, Israel<br />
Introduction. Graft versus host disease (GVHD) still is <strong>the</strong> major<br />
draw-back <strong>of</strong> allogeneic stem cell transplantation (SCT). In its resistant<br />
form, it carries high morbidity and mortality. The mainstay <strong>of</strong><br />
GVHD <strong>the</strong>rapy is preventive and once it is developed <strong>the</strong> first line<br />
<strong>the</strong>rapy is systematically high dose steroids, and cyclosporine or<br />
tacrolimus. It has been shown by us and o<strong>the</strong>r groups, that intra-arterial<br />
targeted steroid <strong>the</strong>rapy may be useful even in steroid refractory<br />
GVHD. Methods. A total 35 patients with 37 cases <strong>of</strong> steroid resistant/dependent<br />
gastrointestinal (GI) (n=16), hepatic (n=15) or combined<br />
(n=6) GVHD, were included and given intra-arterial treatment,<br />
for one or more <strong>of</strong> <strong>the</strong> following arteries - <strong>the</strong> hepatic, gastro-duodenal,<br />
superior mesenteric, inferior mesenteric, internal iliacs. We<br />
defined GI partial response and complete response as <strong>the</strong> day that<br />
symptoms decrease or resolved, respectively. Hepatic partial response<br />
and complete response were defined as <strong>the</strong> day that bilirubin level<br />
began to decrease or decreased below 30% <strong>of</strong> initial level, respectively.<br />
Results. The procedure was safe with no major complications. We<br />
found that Intra-arterial ca<strong>the</strong>ter guided steroid <strong>the</strong>rapy was associated<br />
with partial and complete remission among patients with steroid<br />
resistant or dependent GI or Hepatic GVHD. Hepatic partial response<br />
was observed in 14 (66.6%) patients among whom 7 (33.3%) reached<br />
complete response. GI partial response was observed in 19 (86.4%)<br />
patients among whom 12 (54.4%) reached complete response. An<br />
early administration <strong>of</strong> <strong>the</strong> local <strong>the</strong>rapy, female gender, myeloid basic<br />
disease, and a non-active status <strong>of</strong> <strong>the</strong> basic disease at <strong>the</strong> day <strong>of</strong><br />
transplantation were found related for predicting a better response for<br />
<strong>the</strong> intra-arterial treatment. Conclusions. Intra-arterial ca<strong>the</strong>ter guided<br />
steroid <strong>the</strong>rapy is safe and effective in steroid resistant or dependent<br />
GVHD. A fur<strong>the</strong>r research is warranted characterizing <strong>the</strong> patients<br />
benefit most.<br />
0581<br />
TREATMENT OF CHRONIC EXTENSIVE GRAFT VERSUS HOST DISEASE WITH ALEFACEPT<br />
M.Y. Shapira, I.B. Resnick, P. Tsirigotis, M. Aker, M. Bitan,<br />
B. Gesundheit, S. Slavin, R. Or<br />
Hadassah Hebrew University Medical Cen, JERUSALEM, Israel<br />
Introduction. Alefacept (AMEVIVE ® ) is an immunosuppressive dimeric<br />
fusion protein that consists <strong>of</strong> <strong>the</strong> extracellular CD2-binding portion<br />
<strong>of</strong> <strong>the</strong> human leukocyte function antigen-3 (LFA-3) linked to <strong>the</strong><br />
Fc (hinge, CH2 and CH3 domains) portion <strong>of</strong> human IgG1. We have<br />
recently shown its effect in acute, steroid resistant/dependent GVHD.<br />
In this study, we describe <strong>the</strong> effect <strong>of</strong> alefacept treatment on chronic<br />
extensive graft versus host disease (cGVHD). Patients and Methods.<br />
A total <strong>of</strong> 12 patients (13 cGVHD episodes) were included in this<br />
study, 7 males and 5 females with median age 27 years (range 3-60<br />
years).<br />
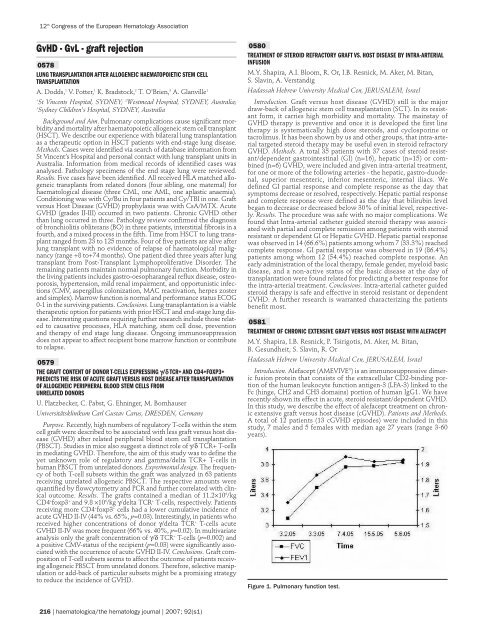
Figure 1. Pulmonary function test.