annual report annual report annual report annual report 2005
annual report annual report annual report annual report 2005
annual report annual report annual report annual report 2005
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
26<br />
RADIATION CHEMISTRY AND PHYSICS, RADIATION TECHNOLOGIES<br />
sible. Besides TP •– , also cation radical and triplet<br />
excited state TP* are known to absorb in the same<br />
region. TEA is a scavenger for cation radical and<br />
excited states of TP. The occurrence of both species<br />
were confirmed also by the experiments under O 2<br />
and N 2 O. Kinetics of the absorption decay is quite<br />
complicated and the best fitting to the experimental<br />
results can be achieved taking an exponential<br />
decay equation with three different constants. In<br />
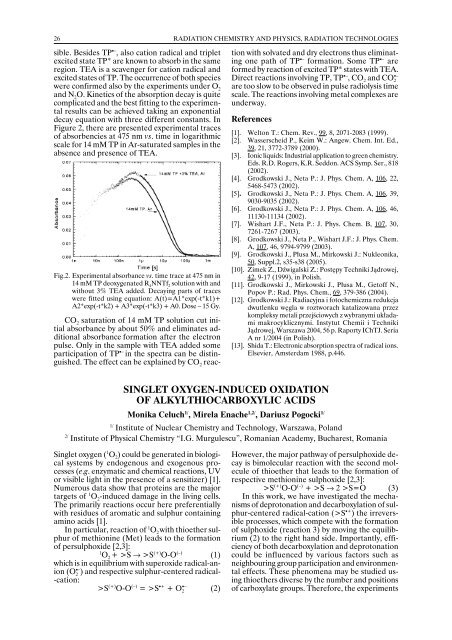
Figure 2, there are presented experimental traces<br />
of absorbencies at 475 nm vs. time in logarithmic<br />
scale for 14 mM TP in Ar-saturated samples in the<br />
absence and presence of TEA.<br />
Fig.2. Experimental absorbance vs. time trace at 475 nm in<br />
14 mM TP deoxygenated R 4<br />
NNTf 2<br />
solution with and<br />
without 3% TEA added. Decaying parts of traces<br />
were fitted using equation: A(t)=A1*exp(-t*k1)+<br />
A2*exp(-t*k2) + A3*exp(-t*k3) + A0. Dose – 15 Gy.<br />
CO 2 saturation of 14 mM TP solution cut initial<br />
absorbance by about 50% and eliminates additional<br />
absorbance formation after the electron<br />
pulse. Only in the sample with TEA added some<br />
participation of TP •– in the spectra can be distinguished.<br />
The effect can be explained by CO 2 reac-<br />
tion with solvated and dry electrons thus eliminating<br />
one path of TP •– formation. Some TP •– are<br />
formed by reaction of excited TP* states with TEA.<br />
Direct reactions involving TP, TP •– , CO 2 and CO 2<br />
•–<br />
are too slow to be observed in pulse radiolysis time<br />
scale. The reactions involving metal complexes are<br />
underway.<br />
References<br />
[1]. Welton T.: Chem. Rev., 99, 8, 2071-2083 (1999).<br />
[2]. Wasserscheid P., Keim W.: Angew. Chem. Int. Ed.,<br />
39, 21, 3772-3789 (2000).<br />
[3]. Ionic liquids: Industrial application to green chemistry.<br />
Eds. R.D. Rogers, K.R. Seddon. ACS Symp. Ser., 818<br />
(2002).<br />
[4]. Grodkowski J., Neta P.: J. Phys. Chem. A, 106, 22,<br />
5468-5473 (2002).<br />
[5]. Grodkowski J., Neta P.: J. Phys. Chem. A, 106, 39,<br />
9030-9035 (2002).<br />
[6]. Grodkowski J., Neta P.: J. Phys. Chem. A, 106, 46,<br />
11130-11134 (2002).<br />
[7]. Wishart J.F., Neta P.: J. Phys. Chem. B, 107, 30,<br />
7261-7267 (2003).<br />
[8]. Grodkowski J., Neta P., Wishart J.F.: J. Phys. Chem.<br />
A, 107, 46, 9794-9799 (2003).<br />
[9]. Grodkowski J., Płusa M., Mirkowski J.: Nukleonika,<br />
50, Suppl.2, s35-s38 (<strong>2005</strong>).<br />
[10]. Zimek Z., Dźwigalski Z.: Postępy Techniki Jądrowej,<br />
42, 9-17 (1999), in Polish.<br />
[11]. Grodkowski J., Mirkowski J., Płusa M., Getoff N.,<br />
Popov P.: Rad. Phys. Chem., 69, 379-386 (2004).<br />
[12]. Grodkowski J.: Radiacyjna i fotochemiczna redukcja<br />
dwutlenku węgla w roztworach katalizowana przez<br />
kompleksy metali przejściowych z wybranymi układami<br />
makrocyklicznymi. Instytut Chemii i Techniki<br />
Jądrowej, Warszawa 2004, 56 p. Raporty IChTJ. Seria<br />
A nr 1/2004 (in Polish).<br />
[13]. Shida T.: Electronic absorption spectra of radical ions.<br />
Elsevier, Amsterdam 1988, p.446.<br />
SINGLET OXYGEN-INDUCED OXIDATION<br />
OF ALKYLTHIOCARBOXYLIC ACIDS<br />
Monika Celuch 1/ , Mirela Enache 1,2/ , Dariusz Pogocki 1/<br />
1/<br />
Institute of Nuclear Chemistry and Technology, Warszawa, Poland<br />
2/<br />
Institute of Physical Chemistry “I.G. Murgulescu”, Romanian Academy, Bucharest, Romania<br />
Singlet oxygen ( 1 O 2 ) could be generated in biological<br />
systems by endogenous and exogenous processes<br />
(e.g. enzymatic and chemical reactions, UV<br />
or visible light in the presence of a sensitizer) [1].<br />
Numerous data show that proteins are the major<br />
targets of 1 O 2<br />
-induced damage in the living cells.<br />
The primarily reactions occur here preferentially<br />
with residues of aromatic and sulphur containing<br />
amino acids [1].<br />
In particular, reaction of 1 O 2 with thioether sulphur<br />
of methionine (Met) leads to the formation<br />
of persulphoxide [2,3]:<br />
1<br />
O 2 + >S → >S (+) O-O (–) (1)<br />
which is in equilibrium with superoxide radical-anion<br />
(O 2<br />
•–<br />
) and respective sulphur-centered radical-<br />
-cation:<br />
>S (+) O-O (–) = >S •+ •–<br />
+ O 2 (2)<br />
However, the major pathway of persulphoxide decay<br />
is bimolecular reaction with the second molecule<br />
of thioether that leads to the formation of<br />
respective methionine sulphoxide [2,3]:<br />
>S (+) O-O (–) + >S → 2 >S=O (3)<br />
In this work, we have investigated the mechanisms<br />
of deprotonation and decarboxylation of sulphur-centered<br />
radical-cation (>S •+ ) the irreversible<br />
processes, which compete with the formation<br />
of sulphoxide (reaction 3) by moving the equilibrium<br />
(2) to the right hand side. Importantly, efficiency<br />
of both decarboxylation and deprotonation<br />
could be influenced by various factors such as<br />
neighbouring group participation and environmental<br />
effects. These phenomena may be studied using<br />
thioethers diverse by the number and positions<br />
of carboxylate groups. Therefore, the experiments