annual report annual report annual report annual report 2005
annual report annual report annual report annual report 2005
annual report annual report annual report annual report 2005
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
RADIOCHEMISTRY, STABLE ISOTOPES,<br />
NUCLEAR ANALYTICAL METHODS, GENERAL CHEMISTRY<br />
THE STRUCTURES OF LEAD(II) COMPLEXES WITH TROPOLONE<br />
Krzysztof Łyczko, Wojciech Starosta<br />
61<br />
Tropolone (2-hydroxy-2,4,6-cycloheptatriene-1-one),<br />
abbreviated as Htrop, is a non-benzenoid aromatic<br />
compound containing a seven-membered ring.<br />
Tropolonato ligand is a bidentate anionic species,<br />
which forms five-membered rings with metal ions<br />
[1]. The functional groups in tropolone (carbonyl<br />
and hydroxyl) make it possible its coordination<br />
to a number of various metal ions. There are<br />
many structural data on homoleptic complexes of<br />
metals with tropolonato ligands, e.g. Cu(trop) 2 [2],<br />
In(trop) 3 [3], Zr(trop) 4 [4]. Some dimeric species,<br />
such as [Tl III (trop)Ph 2 ] 2 [5], [Bi III (trop) 2 (NO 3 )] 2 [6],<br />
[Ni(trop) 2 H 2 O] 2 [7] and polymeric species of Zn 2+<br />
[8] and Hg 2+ [9] with tropolone have also been obtained.<br />
In the dimeric and polymeric compounds<br />
the tropolone plays a bridging role.<br />
Many structural data on coordination compounds<br />
of tropolone with p-block metal cations,<br />
such as gallium, indium [3], thallium(III) [5], tin(II)<br />
[8], tin(IV) [10], bismuth(III) [6] and bismuth(V)<br />
[11] have been <strong>report</strong>ed. The methods of synthesis<br />
of bis(tropolonato)lead(II) and tetrakis(tropolonato)lead(IV)<br />
were described previously [12,13].<br />
Apart from this information, there are no structural<br />
data on any tropolonato-lead compound.<br />
The aim of this work was to determine the structure<br />
of complexes formed by the lead(II) ion and<br />
tropolone molecules. We presumed that the composition<br />
and the structure of the complexes may<br />
depend on the anion. For this purpose, we have<br />
used triflate, perchlorate, nitrate and acetate as<br />
counter ions. The reason was a good solubility of<br />
their lead(II) salts in a water/methanol mixture.<br />
The reaction of tropolone with Pb(CF 3 SO 3 ) 2 ,<br />
Pb(ClO 4 ) 2 , Pb(NO 3 ) 2 and Pb(CH 3 COO) 2 in solution<br />
led to the formation of four different lead(II)<br />
complexes: one dimeric and three polymeric. The<br />
structure of the [Pb(trop)(CF 3 SO 3 )(H 2 O)] n (1),<br />
[Pb 3 (trop) 4 (ClO 4 ) 2 ] n (2), [Pb 2 (trop) 2 (NO 3 ) 2 (CH 3 OH)] n<br />
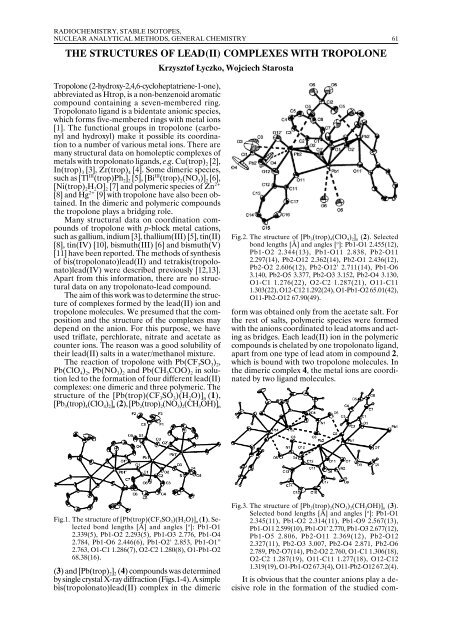
Fig.2. The structure of [Pb 3 (trop) 4 (ClO 4 ) 2 ] n (2). Selected<br />
bond lengths [Å] and angles [ o ]: Pb1-O1 2.455(12),<br />
Pb1-O2 2.344(13), Pb1-O11 2.838, Pb2-O11<br />
2.297(14), Pb2-O12 2.362(14), Pb2-O1 2.436(12),<br />
Pb2-O2 2.606(12), Pb2-O12’ 2.711(14), Pb1-O6<br />
3.140, Pb2-O5 3.377, Pb2-O3 3.152, Pb2-O4 3.130,<br />
O1-C1 1.276(22), O2-C2 1.287(21), O11-C11<br />
1.303(22), O12-C12 1.292(24), O1-Pb1-O2 65.01(42),<br />
O11-Pb2-O12 67.90(49).<br />
form was obtained only from the acetate salt. For<br />
the rest of salts, polymeric species were formed<br />
with the anions coordinated to lead atoms and acting<br />
as bridges. Each lead(II) ion in the polymeric<br />
compounds is chelated by one tropolonato ligand,<br />
apart from one type of lead atom in compound 2,<br />
which is bound with two tropolone molecules. In<br />
the dimeric complex 4, the metal ions are coordinated<br />
by two ligand molecules.<br />
Fig.1. The structure of [Pb(trop)(CF 3 SO 3 )(H 2 O)] n (1). Selected<br />
bond lengths [Å] and angles [ o ]: Pb1-O1<br />
2.339(5), Pb1-O2 2.293(5), Pb1-O3 2.776, Pb1-O4<br />
2.784, Pb1-O6 2.446(6), Pb1-O2’ 2.853, Pb1-O1”<br />
2.763, O1-C1 1.286(7), O2-C2 1.280(8), O1-Pb1-O2<br />
68.38(16).<br />
(3) and [Pb(trop) 2 ] 2 (4) compounds was determined<br />
by single crystal X-ray diffraction (Figs.1-4). A simple<br />
bis(tropolonato)lead(II) complex in the dimeric<br />
Fig.3. The structure of [Pb 2 (trop) 2 (NO 3 ) 2 (CH 3 OH)] n (3).<br />
Selected bond lengths [Å] and angles [ o ]: Pb1-O1<br />
2.345(11), Pb1-O2 2.314(11), Pb1-O9 2.567(13),<br />
Pb1-O11 2.599(10), Pb1-O1’ 2.770, Pb1-O3 2.677(12),<br />
Pb1-O5 2.806, Pb2-O11 2.369(12), Pb2-O12<br />
2.327(11), Pb2-O3 3.007, Pb2-O4 2.871, Pb2-O6<br />
2.789, Pb2-O7(14), Pb2-O2 2.760, O1-C1 1.306(18),<br />
O2-C2 1.287(19), O11-C11 1.277(18), O12-C12<br />
1.319(19), O1-Pb1-O2 67.3(4), O11-Pb2-O12 67.2(4).<br />
It is obvious that the counter anions play a decisive<br />
role in the formation of the studied com-