annual report annual report annual report annual report 2005
annual report annual report annual report annual report 2005
annual report annual report annual report annual report 2005
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
62<br />
RADIOCHEMISTRY, STABLE ISOTOPES,<br />
NUCLEAR ANALYTICAL METHODS, GENERAL CHEMISTRY<br />
pounds through entering (or not entering) the structure.<br />
In compounds 1 and 3, the counter anions<br />
build bridges within the same polymeric chain,<br />
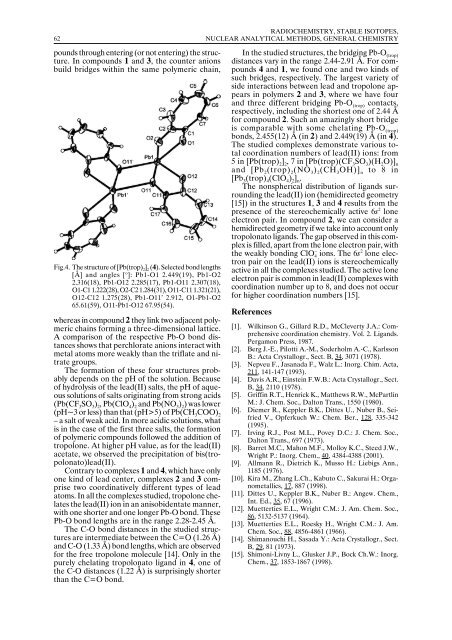
Fig.4. The structure of [Pb(trop) 2<br />
] 2<br />
(4). Selected bond lengths<br />
[Å] and angles [ o ]: Pb1-O1 2.449(19), Pb1-O2<br />
2.316(18), Pb1-O12 2.285(17), Pb1-O11 2.307(18),<br />
O1-C1 1.222(28), O2-C2 1.284(31), O11-C11 1.321(21),<br />
O12-C12 1.275(28), Pb1-O11’ 2.912, O1-Pb1-O2<br />
65.61(59), O11-Pb1-O12 67.95(54).<br />
whereas in compound 2 they link two adjacent polymeric<br />
chains forming a three-dimensional lattice.<br />
A comparison of the respective Pb-O bond distances<br />
shows that perchlorate anions interact with<br />
metal atoms more weakly than the triflate and nitrate<br />
groups.<br />
The formation of these four structures probably<br />
depends on the pH of the solution. Because<br />
of hydrolysis of the lead(II) salts, the pH of aqueous<br />
solutions of salts originating from strong acids<br />
(Pb(CF 3 SO 3 ) 2 , Pb(ClO 4 ) 2 and Pb(NO 3 ) 2 ) was lower<br />
(pH~3 or less) than that (pH>5) of Pb(CH 3 COO) 2<br />
– a salt of weak acid. In more acidic solutions, what<br />
is in the case of the first three salts, the formation<br />
of polymeric compounds followed the addition of<br />
tropolone. At higher pH value, as for the lead(II)<br />
acetate, we observed the precipitation of bis(tropolonato)lead(II).<br />
Contrary to complexes 1 and 4, which have only<br />
one kind of lead center, complexes 2 and 3 comprise<br />
two coordinatively different types of lead<br />
atoms. In all the complexes studied, tropolone chelates<br />
the lead(II) ion in an anisobidentate manner,<br />
with one shorter and one longer Pb-O bond. These<br />
Pb-O bond lengths are in the range 2.28-2.45 Å.<br />
The C-O bond distances in the studied structures<br />
are intermediate between the C=O (1.26 Å)<br />
and C-O (1.33 Å) bond lengths, which are observed<br />
for the free tropolone molecule [14]. Only in the<br />
purely chelating tropolonato ligand in 4, one of<br />
the C-O distances (1.22 Å) is surprisingly shorter<br />
than the C=O bond.<br />
In the studied structures, the bridging Pb-O (trop)<br />
distances vary in the range 2.44-2.91 Å. For compounds<br />
4 and 1, we found one and two kinds of<br />
such bridges, respectively. The largest variety of<br />
side interactions between lead and tropolone appears<br />
in polymers 2 and 3, where we have four<br />
and three different bridging Pb-O (trop) contacts,<br />
respectively, including the shortest one of 2.44 Å<br />
for compound 2. Such an amazingly short bridge<br />
is comparable with some chelating Pb-O (trop)<br />
bonds, 2.455(12) Å (in 2) and 2.449(19) Å (in 4).<br />
The studied complexes demonstrate various total<br />
coordination numbers of lead(II) ions: from<br />
5 in [Pb(trop) 2<br />
] 2<br />
, 7 in [Pb(trop)(CF 3<br />
SO 3<br />
)(H 2<br />
O)] n<br />
and [Pb 2 (trop) 2 (NO 3 ) 2 (CH 3 OH)] n to 8 in<br />
[Pb 3 (trop) 4 (ClO 4 ) 2 ] n .<br />
The nonspherical distribution of ligands surrounding<br />
the lead(II) ion (hemidirected geometry<br />
[15]) in the structures 1, 3 and 4 results from the<br />
presence of the stereochemically active 6s 2 lone<br />
electron pair. In compound 2, we can consider a<br />
hemidirected geometry if we take into account only<br />
tropolonato ligands. The gap observed in this complex<br />
is filled, apart from the lone electron pair, with<br />
the weakly bonding ClO 4– ions. The 6s 2 lone electron<br />
pair on the lead(II) ions is stereochemically<br />
active in all the complexes studied. The active lone<br />
electron pair is common in lead(II) complexes with<br />
coordination number up to 8, and does not occur<br />
for higher coordination numbers [15].<br />
References<br />
[1]. Wilkinson G., Gillard R.D., McCleverty J.A.: Comprehensive<br />
coordination chemistry. Vol. 2. Ligands.<br />
Pergamon Press, 1987.<br />
[2]. Berg J.-E., Pilotti A.-M., Soderholm A.-C., Karlsson<br />
B.: Acta Crystallogr., Sect. B, 34, 3071 (1978).<br />
[3]. Nepveu F., Jasanada F., Walz L.: Inorg. Chim. Acta,<br />
211, 141-147 (1993).<br />
[4]. Davis A.R., Einstein F.W.B.: Acta Crystallogr., Sect.<br />
B, 34, 2110 (1978).<br />
[5]. Griffin R.T., Henrick K., Matthews R.W., McPartlin<br />
M.: J. Chem. Soc., Dalton Trans., 1550 (1980).<br />
[6]. Diemer R., Keppler B.K., Dittes U., Nuber B., Seifried<br />
V., Opferkuch W.: Chem. Ber., 128, 335-342<br />
(1995).<br />
[7]. Irving R.J., Post M.L., Povey D.C.: J. Chem. Soc.,<br />
Dalton Trans., 697 (1973).<br />
[8]. Barret M.C., Mahon M.F., Molloy K.C., Steed J.W.,<br />
Wright P.: Inorg. Chem., 40, 4384-4388 (2001).<br />
[9]. Allmann R., Dietrich K., Musso H.: Liebigs Ann.,<br />
1185 (1976).<br />
[10]. Kira M., Zhang L.Ch., Kabuto C., Sakurai H.: Organometallics,<br />
17, 887 (1998).<br />
[11]. Dittes U., Keppler B.K., Nuber B.: Angew. Chem.,<br />
Int. Ed., 35, 67 (1996).<br />
[12]. Muetterties E.L., Wright C.M.: J. Am. Chem. Soc.,<br />
86, 5132-5137 (1964).<br />
[13]. Muetterties E.L., Roesky H., Wright C.M.: J. Am.<br />
Chem. Soc., 88, 4856-4861 (1966).<br />
[14]. Shimanouchi H., Sasada Y.: Acta Crystallogr., Sect.<br />
B, 29, 81 (1973).<br />
[15]. Shimoni-Livny L., Glusker J.P., Bock Ch.W.: Inorg.<br />
Chem., 37, 1853-1867 (1998).