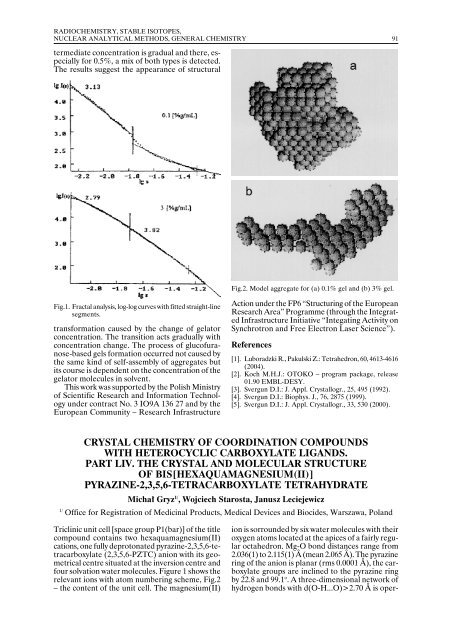
90 RADIOCHEMISTRY, STABLE ISOTOPES, NUCLEAR ANALYTICAL METHODS, GENERAL CHEMISTRY Congress. Part A. Ed. P. Vincenzini. Techna Srl, Faenza 2003, pp.341-352. [2]. Deptuła A., Olczak T., Łada W., Sartowska B., Chmielewski A. G., Alvani C., Carconi P.L., Di Bartolomeo A., Pierdominici F., Casadio S.: J. Sol-Gel Sci. Technol., 26, 207 (2003). [3]. Deptuła A., Olczak T., Łada W., Chmielewski A.G., Alvani C., Carconi P.L., Di Bartolomeo A., Pierdominici F., Casadio S.: J. Mater. Sci., 37, 1 (2002). [4]. Renoult O., Boilot J.-P., Korb J.-P., Boncoeur M.: J. Nucl. Mater., 223, 126 (1995). [5]. Wen Z., Gu Z., Huang S., Yang J., Lin Z., Yamamoto O.: J. Power Sources, 146, 670 (<strong>2005</strong>). [6]. Kavan L., Grätzel M.: Solid State Lett., 5, A39 (2002). [7]. Moshopoulou E.G.: J. Am. Ceram. Soc., 82, 3317 (1999). [8]. Bohnke C., Duroy H., Fourquet J.L.: Sens. Actuators, B89, 240 (2003). [9]. Vijayakumar M., Nghi Pham Q., Bohnke C.: J. Eur. Ceram. Soc., 25, 2973 (<strong>2005</strong>). [10]. Kosewa I., Chaminade J.P., Gravereau P., Pechev S., Pechev P., Etoumeau J.: J. Alloys Compd., 389, 47 (<strong>2005</strong>). [11]. Berbenni V., Marini A.: J. Mater. Sci., 39, 5279 (2004). [12]. Okayama J., Takaya I., Nashimoto K., Sugahara Y.: J. Am. Ceram. Soc., 85, 2195 (2002). [13]. Shendo R., Krueger D.S., Rossetti G.A. Jr., Lombardo S.J.: J. Am. Ceram. Soc., 84, 1648 (2001). [14]. Janes R., Knightley L.J.: J. Mater. Sci., 39, 2589 (2004). [15]. Yang J., Li D., Wang X., Lu L.: J. Mater. Sci., 38, 2907 (2003). [16]. Mazdyiasni K.S, Dolloff R.T., Smith J.: J. Am. Ceram. Soc., 52, 523 (1969). [17]. Phule P.P., Risbud S.H.: Adv. Ceram. Mater., 3, 183 (1988). [18]. Chaput F., Boilot J.P., Beauger A.: J. Am. Ceram. Soc., 73, 942 (1990). [19]. Phule P.P., Risbud S.H.: J. Mater. Sci., 25, 1169 (1990). [20]. Hu M.Z.C., Miller G.A., Payzant E.A., Rawn C.J.: J. Mater. Sci., 35, 2927 (2000). [21]. Cheung M.C., Chan H.L.W., Choy C.L.: J. Mater. Sci., 36, 381 (2001). [22]. Beck H.P., Eisner W., Haberkorn R.: J. Eur. Ceram. Soc., 21, 2319 (2001). [23]. Kumar S., Messing G.L., White W.B.: J. Am. Ceram. Soc., 76, 617 (1993). [24]. Takeuchi T., Tabuchi M., Ado K., Honjo K., Nakamura O., Kageyama H., Suyama Y., Ohtori N., Nagasawa M.: J. Mater. Sci., 32, 4053 (1997). [25]. Deptuła A., Łada W., Olczak T., Lanagan M., Dorris S.E., Goretta K.C., Poeppel R.B.: Polish Patent No. 172618. [26]. Deptuła A., Rebandel J., Drozda W., Łada W., Olczak T.: Mater. Res. Soc. Symp. Proc., 270, 277 (1992). [27]. Deptuła A., Łada W., Olczak T., LeGeros R.Z., LeGeros J.P.: Bioceramics, 9, 313 (1996). [28]. Łada W., Deptuła A., Sartowska B., Olczak T., Chmielewski A.G., Carewska M., Scaccia S., Simonetti E., Giorgi L., Moreno A.: J. New Mater. Electrochem. Syst., 6, 33 (2003). [29]. Chatterjee M., Naskar M.K., Ganguli D.: J. Sol-Gel Sci. Technol., 16, 143 (1999). [30]. Ueyama R., Harada M., Ueyama T., Yamamoto T., Shiosaki T., Seo W.S., Kuribayashi K., Koumoto K.: J. Mater. Sci.: Mater. Electron., 11, 139 (2000). [31]. Holland T.J.B., Redfern S.A.T.: Mineral Mag., 61, 65 (1997). STUDY OF GLUCOFURANOSE-BASED GEL NANOSTRUCTURE USING THE SAXS METHOD Helena Grigoriew, Roman Luboradzki 1/ , Dagmara K. Chmielewska, Monika Mirkowska 2/ 1/ Institute of Physical Chemistry, Polish Academy of Sciences, Warszawa, Poland 2/ Warsaw University of Technology, Poland The glucofuranose-based gels were synthesized by the method described in [1]. The gelator of chemical formula: 1,2-O-(1-ethylpropylidene)-α-D-glucofuranose is built of furanose ring and contains three unprotected -OH groups. Its concentrations of 3, 1, 0.5 and 0.1% in toluene were chosen. The measurements were carried out with a ULTRA-SAXS BW-4 wiggler beamline of the HASYLAB synchrotron. The obtained data were subjected to pie integration and, after normalization, to subtraction of the background which was the SAXS curve of the solvent. For each sample, two measurements with sample-detector distances of 4 and 12 m were performed and joined using OTOKO program [2] Table. Structural parameters vs. gelator concentration. to get a bigger range of the data. The complex method of SAXS data processing was applied, to find structural parameters of the gelator in gel, such as: the mass fractal, d m , and surface fractal, d s , dimensions, radius of gyration, R g , distance distribution function, p(r), and dummy atom models [3-5]. The results (Table and Figs.1 and 2) of all methods make it possible to assume that two types of aggregates exist in the gel. The differences between them are: the first type (for 3% gel) – the aggregate is smaller, compact, of well-defined smooth surface and a rod-like shape, and the second type (for 0.1% gel) – aggregate is bigger, looser, of rough surface and a disk-like shape. The aggregate change at in-
RADIOCHEMISTRY, STABLE ISOTOPES, NUCLEAR ANALYTICAL METHODS, GENERAL CHEMISTRY 91 termediate concentration is gradual and there, especially for 0.5%, a mix of both types is detected. The results suggest the appearance of structural Fig.2. Model aggregate for (a) 0.1% gel and (b) 3% gel. Fig.1. Fractal analysis, log-log curves with fitted straight-line segments. transformation caused by the change of gelator concentration. The transition acts gradually with concentration change. The process of glucofuranose-based gels formation occurred not caused by the same kind of self-assembly of aggregates but its course is dependent on the concentration of the gelator molecules in solvent. This work was supported by the Polish Ministry of Scientific Research and Information Technology under contract No. 3 IO9A 136 27 and by the European Community – Research Infrastructure Action under the FP6 “Structuring of the European Research Area” Programme (through the Integrated Infrastructure Initiative “Integating Activity on Synchrotron and Free Electron Laser Science”). References [1]. Luboradzki R., Pakulski Z.: Tetrahedron, 60, 4613-4616 (2004). [2]. Koch M.H.J.: OTOKO – program package, release 01.90 EMBL-DESY. [3]. Svergun D.I.: J. Appl. Crystallogr., 25, 495 (1992). [4]. Svergun D.I.: Biophys. J., 76, 2875 (1999). [5]. Svergun D.I.: J. Appl. Crystallogr., 33, 530 (2000). CRYSTAL CHEMISTRY OF COORDINATION COMPOUNDS WITH HETEROCYCLIC CARBOXYLATE LIGANDS. PART LIV. THE CRYSTAL AND MOLECULAR STRUCTURE OF BIS[HEXAQUAMAGNESIUM(II)] PYRAZINE-2,3,5,6-TETRACARBOXYLATE TETRAHYDRATE Michał Gryz 1/ , Wojciech Starosta, Janusz Leciejewicz 1/ Office for Registration of Medicinal Products, Medical Devices and Biocides, Warszawa, Poland Triclinic unit cell [space group P1(bar)] of the title compound contains two hexaquamagnesium(II) cations, one fully deprotonated pyrazine-2,3,5,6-tetracarboxylate (2,3,5,6-PZTC) anion with its geometrical centre situated at the inversion centre and four solvation water molecules. Figure 1 shows the relevant ions with atom numbering scheme, Fig.2 – the content of the unit cell. The magnesium(II) ion is sorrounded by six water molecules with their oxygen atoms located at the apices of a fairly regular octahedron. Mg-O bond distances range from 2.036(1) to 2.115(1) Å (mean 2.065 Å). The pyrazine ring of the anion is planar (rms 0.0001 Å), the carboxylate groups are inclined to the pyrazine ring by 22.8 and 99.1 o . A three-dimensional network of hydrogen bonds with d(O-H...O)>2.70 Å is oper-
- Page 1 and 2:
ANNUAL REPORT 2005 50 years in the
- Page 3 and 4:
CONTENTS GENERAL INFORMATION 9 MANA
- Page 5 and 6:
DETERMINATION OF CADMIUM, LEAD, COP
- Page 7 and 8:
NUCLEONIC CONTROL SYSTEMS AND ACCEL
- Page 9 and 10:
GENERAL INFORMATION 9 GENERAL INFOR
- Page 11 and 12:
MANAGEMENT OF THE INSTITUTE 11 MANA
- Page 13 and 14:
MANAGEMENT OF THE INSTITUTE 13 •
- Page 15 and 16:
SCIENTIFIC STAFF 15 5. Danilczuk Ma
- Page 17 and 18:
RADIATION CHEMISTRY AND PHYSICS, RA
- Page 19 and 20:
20 RADIATION CHEMISTRY AND PHYSICS,
- Page 21 and 22:
22 RADIATION CHEMISTRY AND PHYSICS,
- Page 23 and 24:
24 RADIATION CHEMISTRY AND PHYSICS,
- Page 25 and 26:
26 RADIATION CHEMISTRY AND PHYSICS,
- Page 27 and 28:
28 RADIATION CHEMISTRY AND PHYSICS,
- Page 29 and 30:
30 RADIATION CHEMISTRY AND PHYSICS,
- Page 31 and 32:
32 RADIATION CHEMISTRY AND PHYSICS,
- Page 33 and 34:
34 RADIATION CHEMISTRY AND PHYSICS,
- Page 35 and 36:
36 RADIATION CHEMISTRY AND PHYSICS,
- Page 37 and 38: 38 RADIATION CHEMISTRY AND PHYSICS,
- Page 39 and 40: 40 RADIATION CHEMISTRY AND PHYSICS,
- Page 41 and 42: 42 RADIATION CHEMISTRY AND PHYSICS,
- Page 43 and 44: 44 RADIATION CHEMISTRY AND PHYSICS,
- Page 45 and 46: 46 RADIATION CHEMISTRY AND PHYSICS,
- Page 47 and 48: 48 RADIATION CHEMISTRY AND PHYSICS,
- Page 49 and 50: 50 RADIATION CHEMISTRY AND PHYSICS,
- Page 51 and 52: 52 RADIATION CHEMISTRY AND PHYSICS,
- Page 53 and 54: 54 RADIATION CHEMISTRY AND PHYSICS,
- Page 55 and 56: 56 RADIATION CHEMISTRY AND PHYSICS,
- Page 57 and 58: RADIOCHEMISTRY, STABLE ISOTOPES, NU
- Page 59 and 60: RADIOCHEMISTRY, STABLE ISOTOPES, NU
- Page 61 and 62: RADIOCHEMISTRY, STABLE ISOTOPES, NU
- Page 63 and 64: RADIOCHEMISTRY, STABLE ISOTOPES, NU
- Page 65 and 66: RADIOCHEMISTRY, STABLE ISOTOPES, NU
- Page 67 and 68: RADIOCHEMISTRY, STABLE ISOTOPES, NU
- Page 69 and 70: RADIOCHEMISTRY, STABLE ISOTOPES, NU
- Page 71 and 72: RADIOCHEMISTRY, STABLE ISOTOPES, NU
- Page 73 and 74: RADIOCHEMISTRY, STABLE ISOTOPES, NU
- Page 75 and 76: RADIOCHEMISTRY, STABLE ISOTOPES, NU
- Page 77 and 78: RADIOCHEMISTRY, STABLE ISOTOPES, NU
- Page 79 and 80: RADIOCHEMISTRY, STABLE ISOTOPES, NU
- Page 81 and 82: RADIOCHEMISTRY, STABLE ISOTOPES, NU
- Page 83 and 84: RADIOCHEMISTRY, STABLE ISOTOPES, NU
- Page 85 and 86: RADIOCHEMISTRY, STABLE ISOTOPES, NU
- Page 87: RADIOCHEMISTRY, STABLE ISOTOPES, NU
- Page 91 and 92: RADIOCHEMISTRY, STABLE ISOTOPES, NU
- Page 93 and 94: RADIOCHEMISTRY, STABLE ISOTOPES, NU
- Page 95 and 96: RADIOBIOLOGY
- Page 97 and 98: 100 RADIOBIOLOGY THE ROLE OF LYSOSO
- Page 99 and 100: 102 many found the ratio to be high
- Page 101 and 102: 104 RADIOBIOLOGY DNA INTER-STRAND C
- Page 103 and 104: 106 SIRTUIN INHIBITION INCREASES TH
- Page 105 and 106: 108 The possible reason of this eff
- Page 107 and 108: NUCLEAR TECHNOLOGIES AND METHODS 11
- Page 109 and 110: NUCLEAR TECHNOLOGIES AND METHODS 11
- Page 111 and 112: NUCLEAR TECHNOLOGIES AND METHODS 11
- Page 113 and 114: NUCLEAR TECHNOLOGIES AND METHODS 11
- Page 115 and 116: NUCLEAR TECHNOLOGIES AND METHODS 11
- Page 117 and 118: NUCLEAR TECHNOLOGIES AND METHODS 12
- Page 119 and 120: NUCLEAR TECHNOLOGIES AND METHODS 12
- Page 121 and 122: NUCLEAR TECHNOLOGIES AND METHODS 12
- Page 123 and 124: NUCLEAR TECHNOLOGIES AND METHODS 12
- Page 125 and 126: NUCLEAR TECHNOLOGIES AND METHODS 12
- Page 127 and 128: NUCLEAR TECHNOLOGIES AND METHODS 13
- Page 129 and 130: NUCLEAR TECHNOLOGIES AND METHODS 13
- Page 131 and 132: NUCLEAR TECHNOLOGIES AND METHODS 13
- Page 133 and 134: NUCLEAR TECHNOLOGIES AND METHODS 13
- Page 135 and 136: NUCLEAR TECHNOLOGIES AND METHODS 13
- Page 137 and 138: NUCLEAR TECHNOLOGIES AND METHODS 14
- Page 139 and 140:
THE INCT PUBLICATIONS IN 2005 143 T
- Page 141 and 142:
THE INCT PUBLICATIONS IN 2005 145 2
- Page 143 and 144:
THE INCT PUBLICATIONS IN 2005 147 5
- Page 145 and 146:
THE INCT PUBLICATIONS IN 2005 149 8
- Page 147 and 148:
THE INCT PUBLICATIONS IN 2005 CHAPT
- Page 149 and 150:
THE INCT PUBLICATIONS IN 2005 153 2
- Page 151 and 152:
THE INCT PUBLICATIONS IN 2005 155 1
- Page 153 and 154:
THE INCT PUBLICATIONS IN 2005 157 4
- Page 155 and 156:
THE INCT PUBLICATIONS IN 2005 159 6
- Page 157 and 158:
THE INCT PUBLICATIONS IN 2005 161 2
- Page 159 and 160:
THE INCT PUBLICATIONS IN 2005 163 V
- Page 161 and 162:
THE INCT PUBLICATIONS IN 2005 165 6
- Page 163 and 164:
THE INCT PUBLICATIONS IN 2005 167 9
- Page 165 and 166:
NUKLEONIKA 169 NUKLEONIKA THE INTER
- Page 167 and 168:
NUKLEONIKA 171 7. Influence of time
- Page 169 and 170:
INTERVIEWS IN 2005 173 INTERVIEWS I
- Page 171 and 172:
CONFERENCES ORGANIZED AND CO-ORGANI
- Page 173 and 174:
CONFERENCES ORGANIZED AND CO-ORGANI
- Page 175 and 176:
CONFERENCES ORGANIZED AND CO-ORGANI
- Page 177 and 178:
CONFERENCES ORGANIZED AND CO-ORGANI
- Page 179 and 180:
CONFERENCES ORGANIZED AND CO-ORGANI
- Page 181 and 182:
CONFERENCES ORGANIZED AND CO-ORGANI
- Page 183 and 184:
CONFERENCES ORGANIZED AND CO-ORGANI
- Page 185 and 186:
CONFERENCES ORGANIZED AND CO-ORGANI
- Page 187 and 188:
CONFERENCES ORGANIZED AND CO-ORGANI
- Page 189 and 190:
CONFERENCES ORGANIZED AND CO-ORGANI
- Page 191 and 192:
CONFERENCES ORGANIZED AND CO-ORGANI
- Page 193 and 194:
CONFERENCES ORGANIZED AND CO-ORGANI
- Page 195 and 196:
CONFERENCES ORGANIZED AND CO-ORGANI
- Page 197 and 198:
CONFERENCES ORGANIZED AND CO-ORGANI
- Page 199 and 200:
CONFERENCES ORGANIZED AND CO-ORGANI
- Page 201 and 202:
CONFERENCES ORGANIZED AND CO-ORGANI
- Page 203 and 204:
CONFERENCES ORGANIZED AND CO-ORGANI
- Page 205 and 206:
EDUCATION 209 EDUCATION Ph.D. PROGR
- Page 207 and 208:
RESEARCH PROJECTS AND CONTRACTS 211
- Page 209 and 210:
RESEARCH PROJECTS AND CONTRACTS IAE
- Page 211 and 212:
LIST OF VISITORS TO THE INCT IN 200
- Page 213 and 214:
LECTURES AND SEMINARS DELIVERED OUT
- Page 215 and 216:
LECTURES AND SEMINARS DELIVERED OUT
- Page 217 and 218:
AWARDS IN 2005 221 AWARDS IN 2005 1
- Page 219 and 220:
INSTRUMENTAL LABORATORIES AND TECHN
- Page 221 and 222:
INSTRUMENTAL LABORATORIES AND TECHN
- Page 223 and 224:
INSTRUMENTAL LABORATORIES AND TECHN
- Page 225 and 226:
INSTRUMENTAL LABORATORIES AND TECHN
- Page 227 and 228:
INSTRUMENTAL LABORATORIES AND TECHN
- Page 229 and 230:
INSTRUMENTAL LABORATORIES AND TECHN
- Page 231:
INDEX OF THE AUTHORS 235 Lisowska H