annual report annual report annual report annual report 2005
annual report annual report annual report annual report 2005
annual report annual report annual report annual report 2005
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
66<br />
RADIOCHEMISTRY, STABLE ISOTOPES,<br />
NUCLEAR ANALYTICAL METHODS, GENERAL CHEMISTRY<br />
of chemical and physical interactions between the<br />
heavy metals present in solution and functional<br />
groups of the biosorbent: carboxylic, phosphate,<br />
sulfate, ammino, amidic or hydroxylic [10-12].<br />
Divalent cation binding by calcium alginates was<br />
investigated using a number of techniques. Potentiometric<br />
titration revealed that CA exhibits two<br />
distinct pK a<br />
values: (i) similar to that characteristic<br />
of carboxylic groups, and (ii) comparable to that<br />
of saturated alcohols. Esterification of the biosorbent<br />
species resulted in a significant reduction in<br />
metal sorption (up to 10 times), indicating that<br />
mainly carboxylic groups are responsible for the<br />
sorption. Also the ion-selective electrode (ISE)<br />
studies suggest that sorption of cadmium is mainly<br />
due to an ion exchange mechanism [13]. X-ray photoelectron<br />
spectroscopy (XPS) and the infrared<br />
(FTIR) results indicated that both alcoholic (-OH)<br />
and carboxylic (-COO – ) functional groups present<br />
in the carbohydrate moieties play an important role<br />
in the metal removal from aqueous solutions [14].<br />
Because of the difficulties in direct X-ray investigations<br />
of the carbohydrate derivatives, FTIR<br />
absorption spectra of selected M(II)-alginate complexes<br />
and CA bed (Fig.1) were recorded as a continuation<br />
of our studies [15,16] on species formed<br />
by alginic biosorbent with different metal cations.<br />
Vibrational spectroscopy is the most widely used<br />
technique for studying natural products, being fast,<br />
non-destructive, and demanding small sample<br />
amounts. So, the aim of the presented work was to<br />
continue our studies on transition metal complexes<br />
with biosorbents on the alginate origin.<br />
Main vibrational modes: IR spectra of the investigated<br />
species, especially in the fingerprint region,<br />
are rather complex and reflect the complex nature<br />
of the biomass. Analysis of the main features of the<br />
spectra shows that:<br />
- In the fingerprint spectral window all spectra<br />
exhibit the absorbance bands at approximately<br />
1605, 1420, 1085, 1030, 890 and 820 cm –1 . Proposed<br />
assignment of the bands forming the fingerprint<br />
spectral window has been revised since<br />
publication of the previous paper [16] and is presented<br />
in Table 1.<br />
- The difference between the spectra of CA and<br />
M(II)-alginate is mainly in their absorbance intensities.<br />
While the band intensities of the metal-<br />
-loaded CA in the region of the symmetric carboxylate<br />
stretching mode (1450-1300 cm –1 ) are<br />
significantly higher than that of CA, in the asymmetric<br />
carboxylate stretching modes (about 1610<br />
cm –1 ) a slight decrease in the intensity can be<br />
observed for the transition metal cations upon<br />
the exchange of the calcium cation. The absorbance<br />
bands in pure alginic acid are 1740 and<br />
1240 cm –1 .<br />
- The distance between the ν sym and ν asym absorbance<br />
bands for CA is significantly smaller than<br />
those for M(II)-alginate. It can be related to<br />
the higher symmetry in CA in respect to other<br />
M(II)-alginates, which occurred due to complexation<br />
with metal cation.<br />
- The ν asym (COO) band of the M(II)-alginates are<br />
narrower and sharper than that in the CA spec-<br />
trum. It could be linked with orthodox coordination<br />
spheres, wherein both oxygen-M(II)<br />
bonds have a very similar strength.<br />
- Lack of displacement of the bands related to<br />
vibrations associated with the C-O (both alcoholic<br />
and ether groups) at 1085 and 1030 cm –1 is<br />
observed when calcium cation is replaced by another<br />
divalent cation. Such displacement could<br />
result from different coordination strengths of<br />
the metal and calcium cations to both alcoholic<br />
or ether groups.<br />
- Broad absorption peaks in the region of 3250-3500<br />
cm –1 indicate the existence of hydroxyl groups<br />
involved in H-bond network. The peak observed<br />
at 2930 cm –1 can be assigned as vibration of the<br />
CH group [17,18].<br />
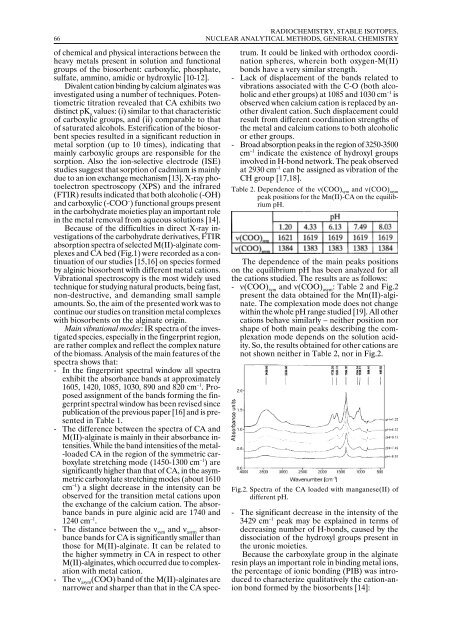
Table 2. Dependence of the ν(COO) sym and ν(COO) asym<br />
peak positions for the Mn(II)-CA on the equilibrium<br />
pH.<br />
The dependence of the main peaks positions<br />
on the equilibrium pH has been analyzed for all<br />
the cations studied. The results are as follows:<br />
- ν(COO) sym<br />
and ν(COO) asym<br />
: Table 2 and Fig.2<br />
present the data obtained for the Mn(II)-alginate.<br />
The complexation mode does not change<br />
within the whole pH range studied [19]. All other<br />
cations behave similarly – neither position nor<br />
shape of both main peaks describing the complexation<br />
mode depends on the solution acidity.<br />
So, the results obtained for other cations are<br />
not shown neither in Table 2, nor in Fig.2.<br />
Fig.2. Spectra of the CA loaded with manganese(II) of<br />
different pH.<br />
- The significant decrease in the intensity of the<br />
3429 cm –1 peak may be explained in terms of<br />
decreasing number of H-bonds, caused by the<br />
dissociation of the hydroxyl groups present in<br />
the uronic moieties.<br />
Because the carboxylate group in the alginate<br />
resin plays an important role in binding metal ions,<br />
the percentage of ionic bonding (PIB) was introduced<br />
to characterize qualitatively the cation-anion<br />
bond formed by the biosorbents [14]: