annual report annual report annual report annual report 2005
annual report annual report annual report annual report 2005
annual report annual report annual report annual report 2005
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
72<br />
RADIOCHEMISTRY, STABLE ISOTOPES,<br />
NUCLEAR ANALYTICAL METHODS, GENERAL CHEMISTRY<br />
last years [1]. These thermodynamically stable and<br />
kinetically inert 99m Tc chelates are good candidates<br />
for radiopharmaceuticals or their precursors. Due<br />
to the softness (HSAB) of the technetium(I) centre,<br />
chelators with soft donor atoms are preferred<br />
as the ligands. Widely studied in this respect are<br />
bi- and tridentate derivatives of pyridine and/or<br />
imidazole (aromatic N donors) in combination with<br />
other donor atoms, in particular sulphur. The aim<br />
of the present work is to select ligands that form<br />
very stable tricarbonyl complexes of technetium(I),<br />
and after further functionalization can be precursors<br />
for radiopharmaceuticals of the second generation.<br />
Two kinds of [Tc(CO) 3 LB] complexes were obtained<br />
and studied, where L is a neutral chelating<br />
ligand with either N,S donor atoms, N-methyl-2-pyridinecarbothioamide,<br />
L NS , or its analog with N,O<br />
donor atoms, N-methyl-2-pyridinecarboamide,<br />
L NO<br />
, while B is a monovalent anion or H 2<br />
O. The<br />
complexes were prepared both with 99m Tc at n.c.a.<br />
level (B=OH – or H 2 O) and with 99 Tc in mg quantities<br />
(B=Cl – ). The 99m Tc complexes were investigated<br />
by HPLC and those of 99 Tc – by IR measurements.<br />
Na[ 99m TcO 4<br />
] was eluted from a 99 Mo/ 99m Tc generator<br />
using 0.9% saline. Synthesis of precursor 1<br />
in water was carried out according to Alberto’s<br />
low-pressure method [2,3] and/or by using potassium<br />
boranocarbonate [4]. The complexes in n.c.a<br />
concentrations were obtained from 1 by adding a<br />
methanol solution of L to the precursor solution<br />
in a phosphate-buffered saline (PBS) to reach<br />
[L]=10 –3 M, followed by heating the mixture at 37<br />
or 75 o C for 10-60 min. The complexes of 99 Tc were<br />
prepared in water-methanol solution by adding<br />
little excess of the ligand to the precursor solution<br />
and heating the mixture at 50 o C, then recrystallized<br />
from a mixture of dichloromethane-hexane.<br />
The IR spectra were carried out in KBr pellets<br />
using a Perkin Elmer 16 PC FT-IR spectrophotometer.<br />
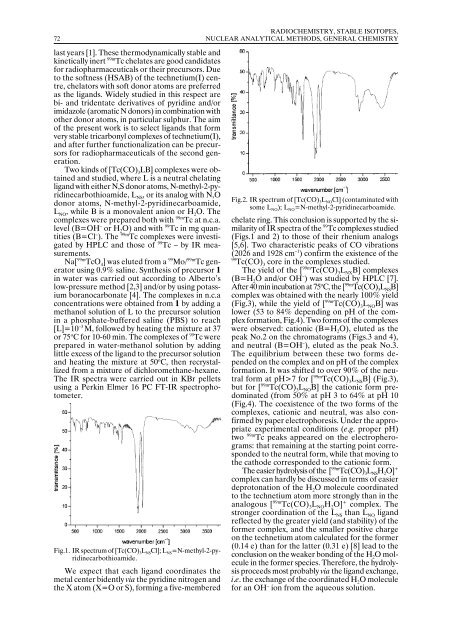
Fig.1. IR spectrum of [Tc(CO) 3 L NS Cl]; L NS =N-methyl-2-pyridinecarbothioamide.<br />
We expect that each ligand coordinates the<br />
metal center bidently via the pyridine nitrogen and<br />
the X atom (X=O or S), forming a five-membered<br />
Fig.2. IR spectrum of [Tc(CO) 3 L NO Cl] (contaminated with<br />
some L NO ); L NO =N-methyl-2-pyridinecarboamide.<br />
chelate ring. This conclusion is supported by the similarity<br />
of IR spectra of the 99 Tc complexes studied<br />
(Figs.1 and 2) to those of their rhenium analogs<br />
[5,6]. Two characteristic peaks of CO vibrations<br />
(2026 and 1928 cm –1 ) confirm the existence of the<br />
99<br />
Tc(CO) 3<br />
core in the complexes studied.<br />
The yield of the [ 99m Tc(CO) 3 L NX B] complexes<br />
(B=H 2 O and/or OH – ) was studied by HPLC [7].<br />
After 40 min incubation at 75 o C, the [ 99m Tc(CO) 3<br />
L NS<br />
B]<br />
complex was obtained with the nearly 100% yield<br />
(Fig.3), while the yield of [ 99m Tc(CO) 3 L NO B] was<br />
lower (53 to 84% depending on pH of the complex<br />
formation, Fig.4). Two forms of the complexes<br />
were observed: cationic (B=H 2 O), eluted as the<br />
peak No.2 on the chromatograms (Figs.3 and 4),<br />
and neutral (B=OH – ), eluted as the peak No.3.<br />
The equilibrium between these two forms depended<br />
on the complex and on pH of the complex<br />
formation. It was shifted to over 90% of the neutral<br />
form at pH>7 for [ 99m Tc(CO) 3 L NS B] (Fig.3),<br />
but for [ 99m Tc(CO) 3 L NO B] the cationic form predominated<br />
(from 50% at pH 3 to 64% at pH 10<br />
(Fig.4). The coexistence of the two forms of the<br />
complexes, cationic and neutral, was also confirmed<br />
by paper electrophoresis. Under the appropriate<br />
experimental conditions (e.g. proper pH)<br />
two 99m Tc peaks appeared on the electropherograms:<br />
that remaining at the starting point corresponded<br />
to the neutral form, while that moving to<br />
the cathode corresponded to the cationic form.<br />
The easier hydrolysis of the [ 99m Tc(CO) 3 L NS H 2 O] +<br />
complex can hardly be discussed in terms of easier<br />
deprotonation of the H 2 O molecule coordinated<br />
to the technetium atom more strongly than in the<br />
analogous [ 99m Tc(CO) 3 L NO H 2 O] + complex. The<br />
stronger coordination of the L NS than L NO ligand<br />
reflected by the greater yield (and stability) of the<br />
former complex, and the smaller positive charge<br />
on the technetium atom calculated for the former<br />
(0.14 e) than for the latter (0.31 e) [8] lead to the<br />
conclusion on the weaker bonding of the H 2 O molecule<br />
in the former species. Therefore, the hydrolysis<br />
proceeds most probably via the ligand exchange,<br />
i.e. the exchange of the coordinated H 2 O molecule<br />
for an OH – ion from the aqueous solution.