Nuclear Energy
Nuclear Energy
Nuclear Energy
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
called enriched uranium. [Note that not all nuclear reactors need enriched uranium, e.g. heavy<br />
water reactors use natural (unenriched) uranium.]<br />
As mentioned above, U-235 also undergoes a small amount of spontaneous fission, which<br />
releases a few free neutrons into any sample of nuclear fuel. One possibility is that such free<br />
neutrons escape rapidly from the fuel mass and decay. That is because free neutrons are unstable,<br />
that is, they are radioactive, each decaying spontaneously, with a half-life of about 15 minutes, into<br />
a proton, an electron and an electron-antineutrino. However, the greater possibility is that these<br />
neutrons collide with other U-235 nuclei in the vicinity, and induce further fissions, releasing yet<br />
more neutrons, thus starting a chain reaction.<br />
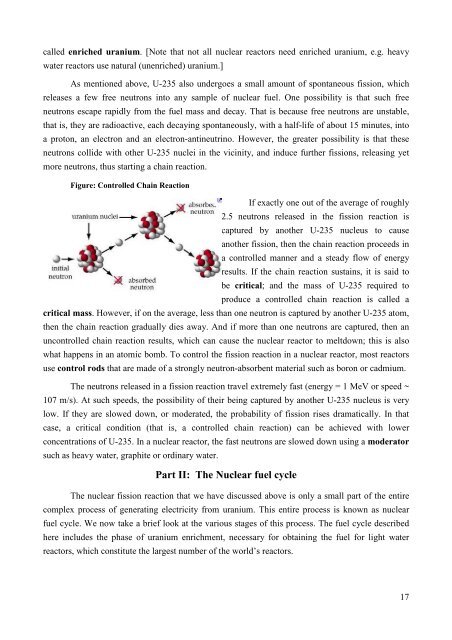
Figure: Controlled Chain Reaction<br />
If exactly one out of the average of roughly<br />
2.5 neutrons released in the fission reaction is<br />
captured by another U-235 nucleus to cause<br />
another fission, then the chain reaction proceeds in<br />
a controlled manner and a steady flow of energy<br />
results. If the chain reaction sustains, it is said to<br />
be critical; and the mass of U-235 required to<br />
produce a controlled chain reaction is called a<br />
critical mass. However, if on the average, less than one neutron is captured by another U-235 atom,<br />
then the chain reaction gradually dies away. And if more than one neutrons are captured, then an<br />
uncontrolled chain reaction results, which can cause the nuclear reactor to meltdown; this is also<br />
what happens in an atomic bomb. To control the fission reaction in a nuclear reactor, most reactors<br />
use control rods that are made of a strongly neutron-absorbent material such as boron or cadmium.<br />
The neutrons released in a fission reaction travel extremely fast (energy = 1 MeV or speed ~<br />
107 m/s). At such speeds, the possibility of their being captured by another U-235 nucleus is very<br />
low. If they are slowed down, or moderated, the probability of fission rises dramatically. In that<br />
case, a critical condition (that is, a controlled chain reaction) can be achieved with lower<br />
concentrations of U-235. In a nuclear reactor, the fast neutrons are slowed down using a moderator<br />
such as heavy water, graphite or ordinary water.<br />
Part II: The <strong>Nuclear</strong> fuel cycle<br />
The nuclear fission reaction that we have discussed above is only a small part of the entire<br />
complex process of generating electricity from uranium. This entire process is known as nuclear<br />
fuel cycle. We now take a brief look at the various stages of this process. The fuel cycle described<br />
here includes the phase of uranium enrichment, necessary for obtaining the fuel for light water<br />
reactors, which constitute the largest number of the world’s reactors.<br />
17