Chapter-1 / Physiological Foundations - WHNLive Public Library
Chapter-1 / Physiological Foundations - WHNLive Public Library
Chapter-1 / Physiological Foundations - WHNLive Public Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
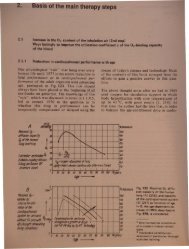
500r-T_-+5__r-_-+1O__,....-_~15.1O-3"'0;/'"blood1lN11A;4~ Bunstn sol"biolyCOffficitnl ~. aO~mIO;lml...""'iI'-+6O'-i--j----t--t---I-------J 55350r--r--+-_-j__-+-~5045range U1!nogO;HT IIuie/(procedure (15min)302550P Oz - ren1501 --r--;.---f---+__+--_-----t-20 range f/JU:i!lgIallfT cureprocedure (J6lJr)1001--rt--t---t---+-----j__--1 15Po;"ven I [f/UidrprOCeriurtl'r---+--tiuring O;HTtcure~tiureo---;;-';;:;:-----:-::::---;:l:=--~--l---...Ja25 as a75 1.0 1.25 15 vol %01~ ~nlrati()nIyoo(19. indiridualsandtiller Oz f1T10 normalorea (arl)oldtr indiriduals5 I ntrlTKJlfOf1(Je (ven)Offer 41fTFig. 7 The physically dissolvedO 2 fraction in the blood as afunction of the arterial (venous)oxygen partial pressure1.1.3 O2 binding curves and exhaustion of the O 2 binding capacity of the bloodAlthough only very small amounts ofO 2 are dissolvedin the blood, the physically dissolved proportionis - as emphasized above - of decisivesignificance for the biological effect and therebyalso for the 02MT, as only the dissolved O 2 moleculescan reach their reactants and thus undergochemical binding.By far the greatest proportion of the oxygentransported with the blood is chemically boundto the hemoglobin of the red blood cells [33].The reaction of the oxygen with the hemoglobinHb + 4 O2 ~ Hb(02)4follows the law of mass action. It is thereforethe physically dissolved O2, which, accordingto Henry' and Dalton's laws, is proportionalto the P02 level, and determines the proportIOnof the hemoglobin to be tran formed intoo yhemoglobin. The proportion of the oxyhmoglobin in th total hemoglobin concentratIOni termed the O 2 saturation of thehemoglobin:[Hb O 2 ]S02=------[Hb] + [Hb O2]This connection between the O 2 partial pre _sure and the O 2 saturation of the hemoglobinis expressed by the O 2 binding curve of theblood. As Fig. 8 shows, th~ curve is S- haped,the steepness being dependent on variou para?1eters (.temperature, pH, PC02)' Fig. 9 givemformatIon about the characteri tic of the O 2b.indi.ng curve (P0 2 range) between O 2 ab orphonm the lung and O 2 delivery to the ti ue inhumans (normal and resting condition ). If, ain accordance with this figure, a mean arterialP02 = 95 mmHg (S02 = 97 %) and a meanvenous P0 2 = 40 mmHg (S02 = 73 %) are a _umed, the content of chemically boundoxygen in the arterial and venou blood an becalculated at about 20 and 15 vol. 0, re pe tively.The arteriovenous difference of the 0concentration is therefore 5 vol. %. 0 n rmallyonly appro imately of one quarter of th t talO2 binding capacity of th blood i ploit d