McKay, Donald. "Front matter" Multimedia Environmental Models ...
McKay, Donald. "Front matter" Multimedia Environmental Models ...
McKay, Donald. "Front matter" Multimedia Environmental Models ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
It is important to note that the reaction rate is controlled by the product V, C,<br />
and k. A large value of any one of these quantities may convey the wrong impression<br />
that the reaction is important.<br />
6.3.5 Level II Using Fugacity and D Values for Reaction<br />
We can now follow the same process as used when treating advection and define<br />
D values for reactions. If the rate is V C k or V Z f k, it is also D Rf, where D R is V<br />
Z k. Note that D R has units of mol/m 3 Pa identical to those of D A or G Z, discussed<br />
earlier. If there are several reactions occurring to the same chemical in the same<br />
phase, then each reaction can be assigned a D value, and these D values can be<br />
added to give a total D value. This is equivalent to adding the rate constants. The<br />
Level II mass balance becomes<br />
©2001 CRC Press LLC<br />
E = SV iC ik i = SV iZ ifk i = fSV iZ ik = fSD R<br />
Thus, f can be deduced, followed by concentrations, amounts, the total amount M,<br />
and the rates of individual reactions as V C k or D f. We can repeat Example 6.2<br />
in fugacity format.<br />
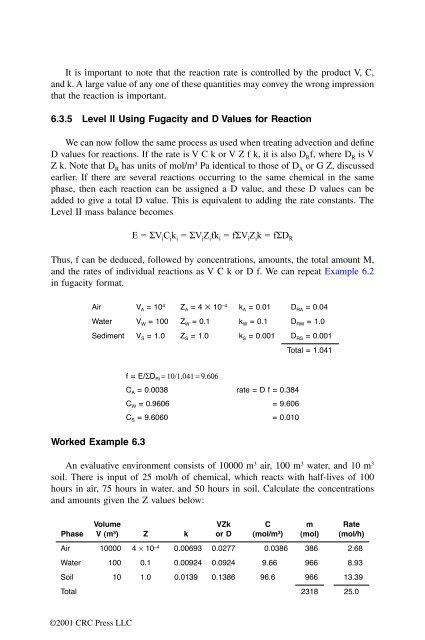
Air V A = 10 4 Z A = 4 ¥ 10 –4 k A = 0.01 D RA = 0.04<br />
Water V W = 100 Z W = 0.1 k W = 0.1 D RW = 1.0<br />
Sediment V S = 1.0 Z S = 1.0 k S = 0.001 D RS = 0.001<br />
Worked Example 6.3<br />
f = E/SD Ri = 10/1.041 = 9.606<br />
C A = 0.0038 rate = D f = 0.384<br />
C W = 0.9606 = 9.606<br />
C S = 9.6060 = 0.010<br />
Total = 1.041<br />
An evaluative environment consists of 10000 m 3 air, 100 m 3 water, and 10 m 3<br />
soil. There is input of 25 mol/h of chemical, which reacts with half-lives of 100<br />
hours in air, 75 hours in water, and 50 hours in soil. Calculate the concentrations<br />
and amounts given the Z values below:<br />
Phase<br />
Volume<br />
V (m 3 ) Z k<br />
VZk<br />
or D<br />
C<br />
(mol/m 3 )<br />
m<br />
(mol)<br />
Rate<br />
(mol/h)<br />
Air 10000 4 ¥ 10 –4 0.00693 0.0277 0.0386 386 2.68<br />
Water 100 0.1 0.00924 0.0924 9.66 966 8.93<br />
Soil 10 1.0 0.0139 0.1386 96.6 966 13.39<br />
Total 2318 25.0