McKay, Donald. "Front matter" Multimedia Environmental Models ...
McKay, Donald. "Front matter" Multimedia Environmental Models ...
McKay, Donald. "Front matter" Multimedia Environmental Models ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
perform a consistency check that, for example, K OA is K OW/K AW. Further checks are<br />
possible if solubilities can be measured to confirm that K AW is S A/S W or P S /S WRT.<br />
These checks are also useful for assessing the “reasonableness” of data. For example,<br />
if an aqueous solubility S W is reported as 1 part per million or 1 g/m 3 or (say)<br />
10 –2 mol/m 3 , and K OW is reported to be 10 7 , then the solubility in octanol must be<br />
S WK OW or 100,000 mol/m 3 . Octanol has a solubility in itself, i.e., a density of about<br />
820 kg/m 3 or 6300 mol/m 3 . It is inconceivable that the solubility of the solute in<br />
octanol exceeds the solubility of octanol in octanol by a factor of 100,000/6300 or<br />
16; therefore, either S W or K OW or both are likely erroneous.<br />
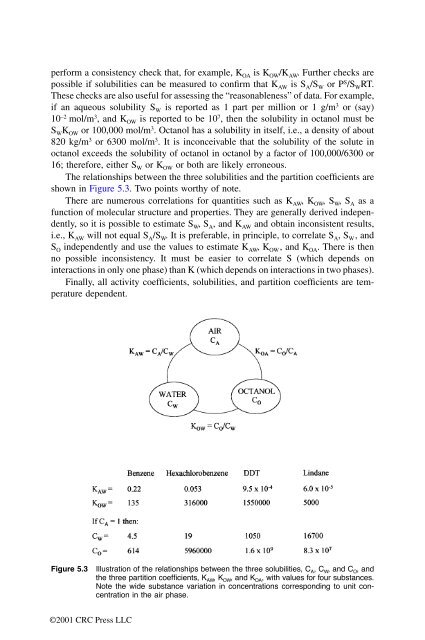
The relationships between the three solubilities and the partition coefficients are<br />
shown in Figure 5.3. Two points worthy of note.<br />
There are numerous correlations for quantities such as K AW, K OW, S W, S A as a<br />
function of molecular structure and properties. They are generally derived independently,<br />
so it is possible to estimate S W, S A, and K AW and obtain inconsistent results,<br />
i.e., K AW will not equal S A/S W. It is preferable, in principle, to correlate S A, S W, and<br />
S O independently and use the values to estimate K AW, K OW, and K OA. There is then<br />
no possible inconsistency. It must be easier to correlate S (which depends on<br />
interactions in only one phase) than K (which depends on interactions in two phases).<br />
Finally, all activity coefficients, solubilities, and partition coefficients are temperature<br />
dependent.<br />
Figure 5.3 Illustration of the relationships between the three solubilities, C A, C W, and C O, and<br />
the three partition coefficients, K AW, K OW, and K OA, with values for four substances.<br />
Note the wide substance variation in concentrations corresponding to unit concentration<br />
in the air phase.<br />
©2001 CRC Press LLC