McKay, Donald. "Front matter" Multimedia Environmental Models ...
McKay, Donald. "Front matter" Multimedia Environmental Models ...
McKay, Donald. "Front matter" Multimedia Environmental Models ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Conversely, if the contact time is short, we can expect to use 4B/pt . If we measure<br />
the transfer rates at several temperatures, and thus different diffusivities, or measure<br />
the transfer rate of different chemicals of different B, then plot kM versus B on loglog<br />
paper, the slope of the line will be 1.0 if steady-state applies, and 0.5 if unsteadystate<br />
applies. In practice, an intermediate power of about 2/3 often applies, suggesting<br />
that we have mostly penetration diffusion followed by a period of near-steady-state<br />
diffusion.<br />
©2001 CRC Press LLC<br />
7.6 DIFFUSION IN POROUS MEDIA<br />
When a solute is diffusing in air or water, its movement is restricted only by<br />
collisions with other molecules. If solid particles or phases are also present, the solid<br />
surfaces will also block diffusion and slow the net velocity. <strong>Environmental</strong>ly, this<br />
is important in sediments in which a solute may have been deposited at some time<br />
in the past, and from which it is now diffusing back to the overlying water. It is also<br />
important in soils from which pesticides may be volatilizing. It is therefore essential<br />
to address the question, “By how much does the presence of the solid phase retard<br />
diffusion?” We assume that the solid particles are in contact, but there remains a<br />
tortuous path for diffusion (otherwise, there is no access route, and the diffusivity<br />
would be zero).<br />
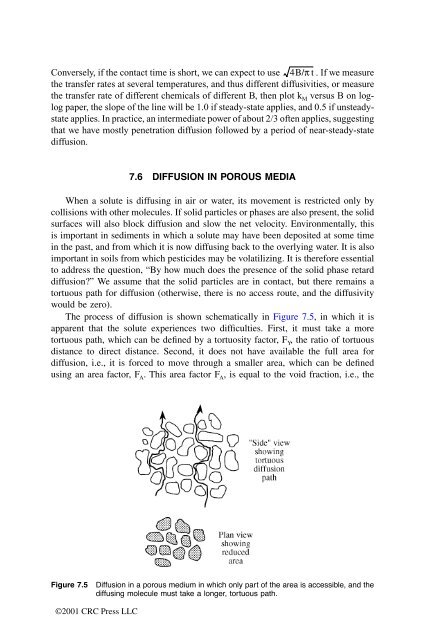
The process of diffusion is shown schematically in Figure 7.5, in which it is<br />
apparent that the solute experiences two difficulties. First, it must take a more<br />
tortuous path, which can be defined by a tortuosity factor, F Y, the ratio of tortuous<br />
distance to direct distance. Second, it does not have available the full area for<br />
diffusion, i.e., it is forced to move through a smaller area, which can be defined<br />
using an area factor, F A. This area factor F A, is equal to the void fraction, i.e., the<br />
Figure 7.5 Diffusion in a porous medium in which only part of the area is accessible, and the<br />
diffusing molecule must take a longer, tortuous path.