McKay, Donald. "Front matter" Multimedia Environmental Models ...
McKay, Donald. "Front matter" Multimedia Environmental Models ...
McKay, Donald. "Front matter" Multimedia Environmental Models ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
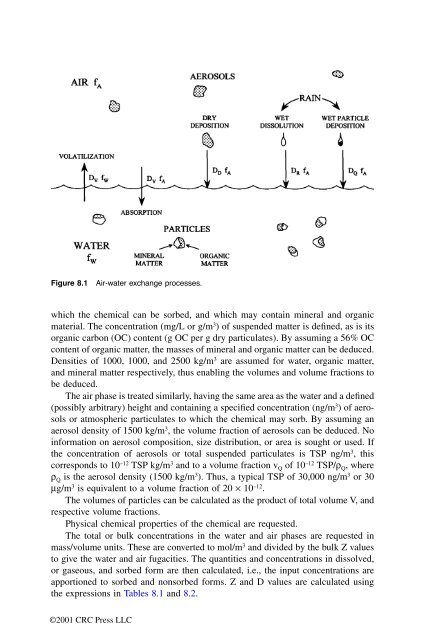
Figure 8.1 Air-water exchange processes.<br />
which the chemical can be sorbed, and which may contain mineral and organic<br />
material. The concentration (mg/L or g/m 3 ) of suspended matter is defined, as is its<br />
organic carbon (OC) content (g OC per g dry particulates). By assuming a 56% OC<br />
content of organic matter, the masses of mineral and organic matter can be deduced.<br />
Densities of 1000, 1000, and 2500 kg/m 3 are assumed for water, organic matter,<br />
and mineral matter respectively, thus enabling the volumes and volume fractions to<br />
be deduced.<br />
The air phase is treated similarly, having the same area as the water and a defined<br />
(possibly arbitrary) height and containing a specified concentration (ng/m 3 ) of aerosols<br />
or atmospheric particulates to which the chemical may sorb. By assuming an<br />
aerosol density of 1500 kg/m 3 , the volume fraction of aerosols can be deduced. No<br />
information on aerosol composition, size distribution, or area is sought or used. If<br />
the concentration of aerosols or total suspended particulates is TSP ng/m 3 , this<br />
corresponds to 10 –12 TSP kg/m 3 and to a volume fraction v Q of 10 –12 TSP/r Q, where<br />
r Q is the aerosol density (1500 kg/m 3 ). Thus, a typical TSP of 30,000 ng/m 3 or 30<br />
mg/m 3 is equivalent to a volume fraction of 20 ¥ 10 –12 .<br />
The volumes of particles can be calculated as the product of total volume V, and<br />
respective volume fractions.<br />
Physical chemical properties of the chemical are requested.<br />
The total or bulk concentrations in the water and air phases are requested in<br />
mass/volume units. These are converted to mol/m 3 and divided by the bulk Z values<br />
to give the water and air fugacities. The quantities and concentrations in dissolved,<br />
or gaseous, and sorbed form are then calculated, i.e., the input concentrations are<br />
apportioned to sorbed and nonsorbed forms. Z and D values are calculated using<br />
the expressions in Tables 8.1 and 8.2.<br />
©2001 CRC Press LLC