McKay, Donald. "Front matter" Multimedia Environmental Models ...
McKay, Donald. "Front matter" Multimedia Environmental Models ...
McKay, Donald. "Front matter" Multimedia Environmental Models ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Another important classification of organic chemicals is according to their dissociating<br />
tendencies in water solution. Some organic acids, notably the phenols, will<br />
form ionic species (phenolates) at high pH. The tendency to ionize is characterized<br />
by the acid dissociation constant KA,<br />
often expressed as pKA,<br />
its negative base ten<br />
logarithm.<br />
In concert with partitioning characteristics, the other set of properties that determine<br />
environmental behavior is reactivity or persistence, usually expressed as a halflife.<br />
It is misleading to assign a single number to a half-life, because it depends on<br />
the intrinsic properties of the chemical and on the nature of the environment. Factors<br />
such as sunlight intensity, hydroxyl radical concentration, the nature of the microbial<br />
community, as well as temperature vary considerably from place to place and time<br />
to time. Here, we use a semiquantitative classification of half-lives into classes,<br />
assuming that average environmental conditions apply. Different classes are defined<br />
for air, water, soils, and sediments. The classification is that used in a series of<br />
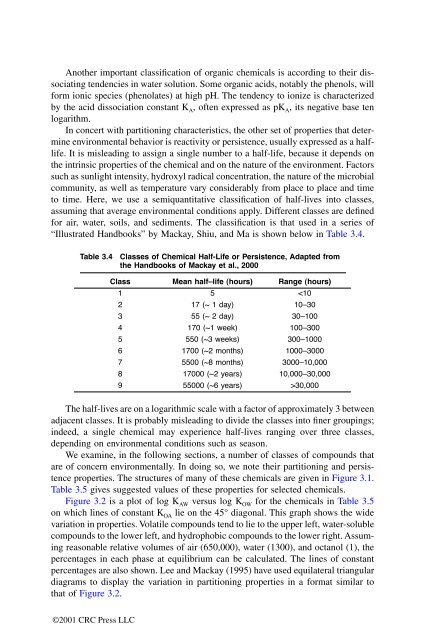
“Illustrated Handbooks” by Mackay, Shiu, and Ma is shown below in Table 3.4.<br />
Table 3.4 Classes of Chemical Half-Life or Persistence, Adapted from<br />
the Handbooks of Mackay et al., 2000<br />
The half-lives are on a logarithmic scale with a factor of approximately 3 between<br />
adjacent classes. It is probably misleading to divide the classes into finer groupings;<br />
indeed, a single chemical may experience half-lives ranging over three classes,<br />
depending on environmental conditions such as season.<br />
We examine, in the following sections, a number of classes of compounds that<br />
are of concern environmentally. In doing so, we note their partitioning and persistence<br />
properties. The structures of many of these chemicals are given in Figure 3.1.<br />
Table 3.5 gives suggested values of these properties for selected chemicals.<br />
Figure 3.2 is a plot of log KAW<br />
versus log KOW<br />
for the chemicals in Table 3.5<br />
on which lines of constant KOA<br />
lie on the 45° diagonal. This graph shows the wide<br />
variation in properties. Volatile compounds tend to lie to the upper left, water-soluble<br />
compounds to the lower left, and hydrophobic compounds to the lower right. Assuming<br />
reasonable relative volumes of air (650,000), water (1300), and octanol (1), the<br />
percentages in each phase at equilibrium can be calculated. The lines of constant<br />
percentages are also shown. Lee and Mackay (1995) have used equilateral triangular<br />
diagrams to display the variation in partitioning properties in a format similar to<br />
that of Figure 3.2.<br />
©2001 CRC Press LLC<br />
Class Mean half–life (hours) Range (hours)<br />
1 5 30,000