APPENDICES. A systematic review and economic model of the ...
APPENDICES. A systematic review and economic model of the ...
APPENDICES. A systematic review and economic model of the ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
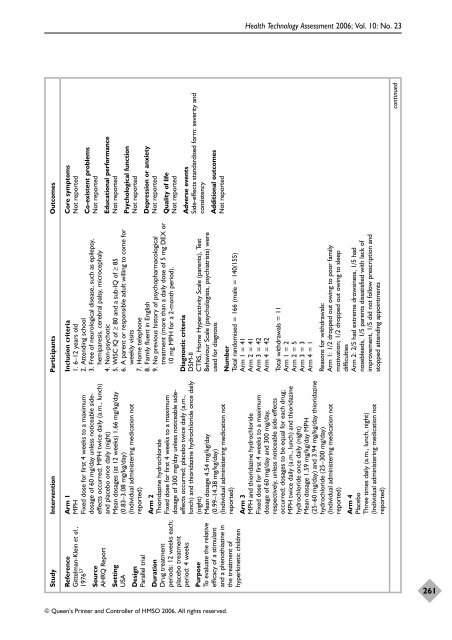
Study Intervention Participants Outcomes<br />
Core symptoms<br />
Not reported<br />
Reference<br />
Gittelman-Klein et al.,<br />
1976 57<br />
Co-existent problems<br />
Not reported<br />
Source<br />
AHRQ Report<br />
Educational performance<br />
Not reported<br />
Setting<br />
USA<br />
Psychological function<br />
Not reported<br />
Arm 1<br />
MPH<br />
Fixed dose for first 4 weeks to a maximum<br />
dosage <strong>of</strong> 60 mg/day unless noticeable sideeffects<br />
occurred; MPH twice daily (a.m., lunch)<br />
<strong>and</strong> placebo once daily (night)<br />
Mean dosages (at 12 weeks) 1.66 mg/kg/day<br />
(0.83–3.08 mg/kg/day)<br />
(Individual administering medication not<br />
reported)<br />
Design<br />
Parallel trial<br />
Depression or anxiety<br />
Not reported<br />
Inclusion criteria<br />
1. 6–12 years old<br />
2. Attending school<br />
3. Free <strong>of</strong> neurological disease, such as epilepsy,<br />
hemiparesis, cerebral palsy, microcephaly<br />
4. Non-psychotic<br />
5. WISC IQ <strong>of</strong> ≥ 80 <strong>and</strong> a sub-IQ <strong>of</strong> ≥ 85<br />
6. A parent or responsible adult willing to come for<br />
weekly visits<br />
7. Home telephone<br />
8. Family fluent in English<br />
9. No previous history <strong>of</strong> psychopharmacological<br />
treatment (more than a daily dose <strong>of</strong> 5 mg DEX or<br />
10 mg MPH for a 2-month period).<br />
Quality <strong>of</strong> life<br />
Not reported<br />
Adverse events<br />
Side-effects st<strong>and</strong>ardised form: severity <strong>and</strong><br />
consistency<br />
Arm 2<br />
Thioridazine hydrochloride<br />
Fixed dose for first 4 weeks to a maximum<br />
dosage <strong>of</strong> 300 mg/day unless noticeable sideeffects<br />
occurred; placebo twice daily (a.m.,<br />
lunch) <strong>and</strong> thioridazine hydrochloride once daily<br />
(night)<br />
Mean dosage 4.54 mg/kg/day<br />
(0.99–14.38 mg/kg/day)<br />
(Individual administering medication not<br />
reported)<br />
Duration<br />
Drug treatment<br />
periods: 12 weeks each;<br />
placebo treatment<br />
period: 4 weeks<br />
Additional outcomes<br />
Not reported<br />
Diagnostic criteria<br />
DSM-II<br />
CTRS, Home Hyperactivity Scale (parents), Test<br />
Behaviour Scale (psychologists, psychiatrists) were<br />
used for diagnosis<br />
Purpose<br />
To evaluate <strong>the</strong> relative<br />
efficacy <strong>of</strong> a stimulant<br />
<strong>and</strong> a phenothiazine in<br />
<strong>the</strong> treatment <strong>of</strong><br />
hyperkinetic children<br />
© Queen’s Printer <strong>and</strong> Controller <strong>of</strong> HMSO 2006. All rights reserved.<br />
Health Technology Assessment 2006; Vol. 10: No. 23<br />
Number<br />
Total r<strong>and</strong>omised = 166 (male = 140/155)<br />
Arm 1 = 41<br />
Arm 2 = 41<br />
Arm 3 = 42<br />
Arm 4 = 42<br />
Total withdrawals = 11<br />
Arm 1 = 2<br />
Arm 2 = 5<br />
Arm 3 = 3<br />
Arm 4 = 1<br />
Reasons for withdrawals:<br />
Arm 1: 1/2 dropped out owing to poor family<br />
motivation; 1/2 dropped out owing to sleep<br />
difficulties<br />
Arm 2: 2/5 had extreme drowsiness, 1/5 had<br />
nosebleeds, 1/5 parents dissatisfied with lack <strong>of</strong><br />
improvement, 1/5 did not follow prescription <strong>and</strong><br />
stopped attending appointments<br />
Arm 3<br />
MPH <strong>and</strong> thioridazine hydrochloride<br />
Fixed dose for first 4 weeks to a maximum<br />
dosage <strong>of</strong> 60 mg/day <strong>and</strong> 300 mg/day,<br />
respectively, unless noticeable side-effects<br />
occurred; dosages to be equal for each drug;<br />
MPH twice daily (a.m., lunch) <strong>and</strong> thioridazine<br />
hydrochloride once daily (night)<br />
Mean dosage 1.59 mg/kg/day MPH<br />
(25–60 mg/day) <strong>and</strong> 3.94 mg/kg/day thioridazine<br />
hydrochloride (25–300 mg/day)<br />
(Individual administering medication not<br />
reported)<br />
Arm 4<br />
Placebo<br />
Three times daily (a.m., lunch, night)<br />
(Individual administering medication not<br />
reported)<br />
continued<br />
261