APPENDICES. A systematic review and economic model of the ...
APPENDICES. A systematic review and economic model of the ...
APPENDICES. A systematic review and economic model of the ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
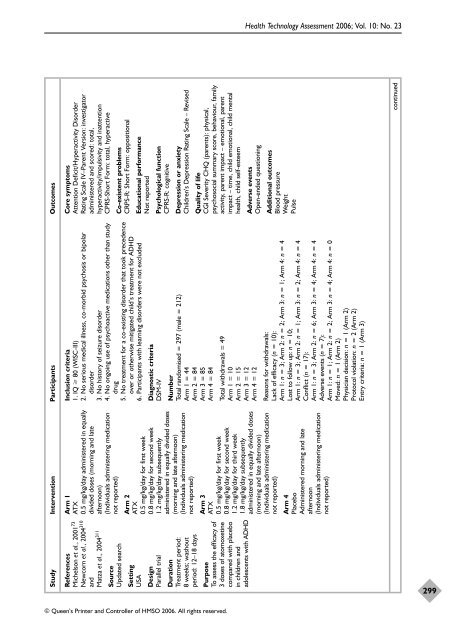
Study Intervention Participants Outcomes<br />
Core symptoms<br />
Attention Deficit/Hyperactivity Disorder<br />
Rating Scale IV–Parent Version: investigator<br />
administered <strong>and</strong> scored: total,<br />
hyperactivity/impulsivity <strong>and</strong> inattention<br />
CPRS-Short Form: total, hyperactive<br />
Arm 1<br />
ATX<br />
0.5 mg/kg/day administered in equally<br />
divided doses (morning <strong>and</strong> late<br />
afternoon)<br />
(Individuals administering medication<br />
not reported)<br />
References<br />
Michelson et al., 2001 73<br />
Newcorn et al., 2004 310<br />
<strong>and</strong><br />
Matza et al., 2004 311<br />
Co-existent problems<br />
CRPS-R: Short Form: oppositional<br />
Source<br />
Updated search<br />
Educational performance<br />
Not reported<br />
Inclusion criteria<br />
1. IQ 80 (WISC-III)<br />
2. No serious medical illness, co-morbid psychosis or bipolar<br />
disorder<br />
3. No history <strong>of</strong> seizure disorder<br />
4. No ongoing use <strong>of</strong> psychoactive medications o<strong>the</strong>r than study<br />
drug<br />
5. No treatment for a co-existing disorder that took precedence<br />
over or o<strong>the</strong>rwise mitigated child’s treatment for ADHD<br />
6. Participants with learning disorders were not excluded<br />
Setting<br />
USA<br />
Psychological function<br />
CPRS-R: cognitive<br />
Arm 2<br />
ATX<br />
0.5 mg/kg/day for first week<br />
0.8 mg/kg/day for second week<br />
1.2 mg/kg/day subsequently<br />
administered in equally divided doses<br />
(morning <strong>and</strong> late afternoon)<br />
(Individuals administering medication<br />
not reported)<br />
Design<br />
Parallel trial<br />
Depression or anxiety<br />
Children’s Depression Rating Scale – Revised<br />
Diagnostic criteria<br />
DSM-IV<br />
Number<br />
Total r<strong>and</strong>omised = 297 (male = 212)<br />
Arm 1 = 44<br />
Arm 2 = 84<br />
Arm 3 = 85<br />
Arm 4 = 84<br />
Duration<br />
Treatment period:<br />
8 weeks; washout<br />
period: 12–18 days<br />
Quality <strong>of</strong> life<br />
CGI Severity CHQ (parents): physical,<br />
psychosocial summary score, behaviour, family<br />
activity, parent impact – emotional, parent<br />
impact – time, child emotional, child mental<br />
health, child self-esteem<br />
Purpose<br />
To assess <strong>the</strong> efficacy <strong>of</strong><br />
3 doses <strong>of</strong> atomoxetine<br />
compared with placebo<br />
in children <strong>and</strong><br />
adolescents with ADHD<br />
© Queen’s Printer <strong>and</strong> Controller <strong>of</strong> HMSO 2006. All rights reserved.<br />
Total withdrawals = 49<br />
Arm 1 = 10<br />
Arm 2 = 15<br />
Arm 3 = 12<br />
Arm 4 = 12<br />
Arm 3<br />
ATX<br />
0.5 mg/kg/day for first week<br />
0.8 mg/kg/day for second week<br />
1.2 mg/kg/day for third week<br />
1.8 mg/kg/day subsequently<br />
administered in equally divided doses<br />
(morning <strong>and</strong> late afternoon)<br />
(Individuals administering medication<br />
not reported)<br />
Health Technology Assessment 2006; Vol. 10: No. 23<br />
Adverse events<br />
Open-ended questioning<br />
Additional outcomes<br />
Blood pressure<br />
Weight<br />
Pulse<br />
Reasons for withdrawals:<br />
Lack <strong>of</strong> efficacy (n = 10):<br />
Arm 1: n = 3; Arm 2: n = 2; Arm 3: n = 1; Arm 4: n = 4<br />
Lost to follow up: n = 10;<br />
Arm 1: n = 3; Arm 2: n = 1; Arm 3: n = 2; Arm 4: n = 4<br />
Conflict (n = 17):<br />
Arm 1: n = 3; Arm 2: n = 6; Arm 3: n = 4; Arm 4: n = 4<br />
Adverse events (n = 7):<br />
Arm 1: n = 1; Arm 2: n = 2; Arm 3: n = 4; Arm 4: n = 0<br />
Moved: n = 1 (Arm 2)<br />
Physician decision: n = 1 (Arm 2)<br />
Protocol violation: n = 2 (Arm 2)<br />
Entry criteria: n = 1 (Arm 3)<br />
Arm 4<br />
Placebo<br />
Administered morning <strong>and</strong> late<br />
afternoon<br />
(Individuals administering medication<br />
not reported)<br />
continued<br />
299