You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
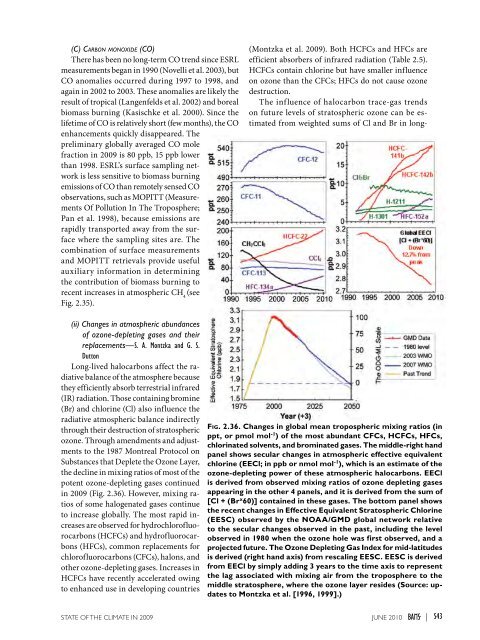
(C) Carbon monoxide (CO)There has been no long-term CO trend since ESRLmeasurements began in 1990 (Novelli et al. 2003), butCO anomalies occurred during 1997 to 1998, andagain in 2002 to 2003. These anomalies are likely theresult of tropical (Langenfelds et al. 2002) and borealbiomass burning (Kasischke et al. 2000). Since thelifetime of CO is relatively short (few months), the COenhancements quickly disappeared. Thepreliminary globally averaged CO molefraction in <strong>2009</strong> is 80 ppb, 15 ppb lowerthan 1998. ESRL’s surface sampling networkis less sensitive to biomass burningemissions of CO than remotely sensed COobservations, such as MOPITT (MeasurementsOf Pollution In The Troposphere;Pan et al. 1998), because emissions arerapidly transported away from the surfacewhere the sampling sites are. Thecombination of surface measurementsand MOPITT retrievals provide usefulauxiliary information in determiningthe contribution of biomass burning torecent increases in atmospheric CH 4(seeFig. 2.35).(Montzka et al. <strong>2009</strong>). Both HCFCs and HFCs areefficient absorbers of infrared radiation (Table 2.5).HCFCs contain chlorine but have smaller influenceon ozone than the CFCs; HFCs do not cause ozonedestruction.The influence of halocarbon trace-gas trendson future levels of stratospheric ozone can be estimatedfrom weighted sums of Cl and Br in long-(ii) Changes in atmospheric abundancesof ozone-depleting gases and theirreplacements—S. A. Montzka and G. S.DuttonLong-lived halocarbons affect the radiativebalance of the atmosphere becausethey efficiently absorb terrestrial infrared(IR) radiation. Those containing bromine(Br) and chlorine (Cl) also influence theradiative atmospheric balance indirectlythrough their destruction of stratosphericozone. Through amendments and adjustmentsto the 1987 Montreal Protocol onSubstances that Deplete the Ozone Layer,the decline in mixing ratios of most of thepotent ozone-depleting gases continuedin <strong>2009</strong> (Fig. 2.36). However, mixing ratiosof some halogenated gases continueto increase globally. The most rapid increasesare observed for hydrochlorofluorocarbons(HCFCs) and hydrofluorocarbons(HFCs), common replacements forchlorofluorocarbons (CFCs), halons, andother ozone-depleting gases. Increases inHCFCs have recently accelerated owingto enhanced use in developing countriesFig. 2.36. Changes in global mean tropospheric mixing ratios (inppt, or pmol mol -1 ) of the most abundant CFCs, HCFCs, HFCs,chlorinated solvents, and brominated gases. The middle-right handpanel shows secular changes in atmospheric effective equivalentchlorine (EECl; in ppb or nmol mol -1 ), which is an estimate of theozone-depleting power of these atmospheric halocarbons. EEClis derived from observed mixing ratios of ozone depleting gasesappearing in the other 4 panels, and it is derived from the sum of[Cl + (Br x 60)] contained in these gases. The bottom panel showsthe recent changes in Effective Equivalent Stratospheric Chlorine(EESC) observed by the NOAA/GMD global network relativeto the secular changes observed in the past, including the levelobserved in 1980 when the ozone hole was first observed, and aprojected future. The Ozone Depleting Gas Index for mid-latitudesis derived (right hand axis) from rescaling EESC. EESC is derivedfrom EECl by simply adding 3 years to the time axis to representthe lag associated with mixing air from the troposphere to themiddle stratosphere, where the ozone layer resides (Source: updatesto Montzka et al. [1996, 1999].)<strong>STATE</strong> <strong>OF</strong> <strong>THE</strong> <strong>CLIMATE</strong> <strong>IN</strong> <strong>2009</strong> juNE 2010 |S43