Landfills and waste water treatment plants as sources of ... - GKSS
Landfills and waste water treatment plants as sources of ... - GKSS
Landfills and waste water treatment plants as sources of ... - GKSS
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
INTRODUCTION<br />
several studies observed those compounds predominantly in the g<strong>as</strong> ph<strong>as</strong>e (Peck <strong>and</strong><br />
Hornbuckle 2004; Xie et al. 2007).<br />
A substance is bioaccumulative with a log KOW value greater than 5 (UNEP 2010). On the<br />
b<strong>as</strong>is <strong>of</strong> this criterion, almost all substances <strong>of</strong> this study are <strong>as</strong>sumed to be bioaccumulative.<br />
Due to the amphipilic character <strong>of</strong> ionic PFCs it is not possible to determine their KOW.<br />
However, their potential <strong>of</strong> bioaccumulation h<strong>as</strong> been demonstrated (Conder et al. 2008).<br />
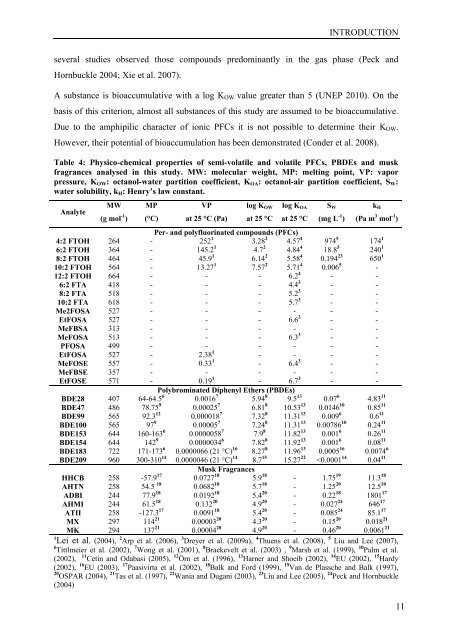
Table 4: Physico-chemical properties <strong>of</strong> semi-volatile <strong>and</strong> volatile PFCs, PBDEs <strong>and</strong> musk<br />
fragrances analysed in this study. MW: molecular weight, MP: melting point, VP: vapor<br />
pressure, KOW: octanol-<strong>water</strong> partition coefficient, KOA: octanol-air partition coefficient, SW:<br />
<strong>water</strong> solubility, kH: Henry’s law constant.<br />
Analyte<br />
MW<br />
(g mol -1 )<br />
MP<br />
(°C)<br />
VP<br />
at 25 °C (Pa)<br />
log KOW<br />
at 25 °C<br />
log KOA<br />
at 25 °C<br />
SW<br />
(mg L -1 )<br />
kH<br />
(Pa m 3 mol -1 )<br />
Per- <strong>and</strong> polyfluorinated compounds (PFCs)<br />
4:2 FTOH 264 - 252 1 3.28 2 4.57 4 974 5 174 1<br />
6:2 FTOH 364 - 145.2 1 4.7 2 4.84 4 18.8 5 240 1<br />
8:2 FTOH 464 - 45.9 1 6.14 2 5.58 4 0.194 23 650 1<br />
10:2 FTOH 564 - 13.27 1 7.57 2 5.71 4 0.006 5 -<br />
12:2 FTOH 664 - - - 6.2 4 - -<br />
6:2 FTA 418 - - - 4.4 3 - -<br />
8:2 FTA 518 - - - 5.2 3 - -<br />
10:2 FTA 618 - - - 5.7 3 - -<br />
Me2FOSA 527 - - - - - -<br />
EtFOSA 527 - - - 6.6 3 - -<br />
MeFBSA 313 - - - - - -<br />
MeFOSA 513 - - - 6.3 3 - -<br />
PFOSA 499 - - - - - -<br />
EtFOSA 527 - 2.38 1 - - - -<br />
MeFOSE 557 - 0.33 1 - 6.4 3 - -<br />
MeFBSE 357 - - - - - -<br />
EtFOSE 571 - 0.19 1 - 6.7 3 - -<br />
Polybrominated Diphenyl Ethers (PBDEs)<br />
BDE28 407 64-64.5 6 0.0016 7 5.94 8 9.5 13 0.07 6 4.83 11<br />
BDE47 486 78.75 9 0.00025 7 6.81 8 10.53 13 0.0146 10 0.85 11<br />
BDE99 565 92.3 12 0.000018 7 7.32 8 11.31 13 0.009 6 0.6 11<br />
BDE100 565 97 9 0.00005 7 7.24 8 11.31 13 0.00786 10 0.24 11<br />
BDE153 644 160-163 6 0.0000058 7 7.9 8 11.82 13 0.001 6 0.26 11<br />
BDE154 644 142 9 0.0000034 6 7.82 8 11.92 13 0.001 6 0.08 11<br />
BDE183 722 171-173 6 0.0000066 (21 °C) 16 8.27 8 11.96 13 0.0005 16 0.0074 6<br />
BDE209 960 300-310 14 0.0000046 (21 °C) 14 8.7 15 15.27 22