Business Potential for Agricultural Biotechnology - Asian Productivity ...
Business Potential for Agricultural Biotechnology - Asian Productivity ...
Business Potential for Agricultural Biotechnology - Asian Productivity ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Agricultural</strong><br />
research<br />
contains<br />
more biotechnology<br />
components<br />
Management<br />
mechanisms<br />
in place to<br />
control food<br />
safety and<br />
inspection<br />
Host of<br />
shrimp<br />
cluster identified<br />
A clear policy<br />
toward<br />
GMOs is<br />
established<br />
Mutual cooperation<br />
among organizations<br />
under different<br />
authorities<br />
Database<br />
network on<br />
food and<br />
safety established<br />
Service business<br />
on food<br />
/seed inspection<br />
<strong>for</strong> export<br />
emerges<br />
Widespread<br />
use of DNA<br />
technology<br />
<strong>for</strong> plant and<br />
livestock<br />
breeding<br />
Organization<br />
established<br />
to handle in<strong>for</strong>mation<br />
<strong>for</strong> trade-relateddecision-making<br />
and negotiation<br />
Rice and<br />
cassava<br />
clusters <strong>for</strong>mulated<br />
<strong>Business</strong> <strong>Potential</strong> <strong>for</strong> <strong>Agricultural</strong> <strong>Biotechnology</strong> Products in Thailand<br />
Have proficiency<br />
in<br />
food risk<br />
assessment<br />
Domesticate<br />
of shrimp<br />
broodstocks<br />
occupy 50%<br />
of total supply<br />
Post-harvest<br />
and packagingtechnologiesutilized<br />
to expandvegetable/fruit<br />
market<br />
Food researchinstituteestablished<br />
25% of exportedvegetables/fruits<br />
utilize biocontrol<br />
<strong>for</strong><br />
pest management<br />
– 179 –<br />
A policy<br />
shift from<br />
the country’s<br />
role as OEM<br />
in seed production<br />
to<br />
that of developer<br />
and<br />
exporter<br />
New bestseller<br />
marine<br />
products in<br />
addition to<br />
shrimp<br />
Thai livestockproductsaccepted<br />
in global<br />
market<br />
Export of<br />
processed<br />
agricultural<br />
products<br />
ranked<br />
global top 5<br />
Total food<br />
export value<br />
reaches THB<br />
1.2 trillion<br />
<strong>Biotechnology</strong><br />
helped<br />
to promote<br />
Thailand as<br />
kitchen of<br />
the world<br />
2004 2005 2006 2007 2008 2009 2010 2011<br />
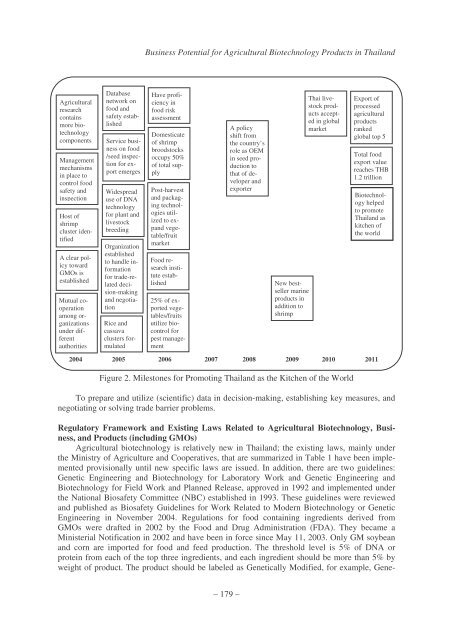
Figure 2. Milestones <strong>for</strong> Promoting Thailand as the Kitchen of the World<br />
To prepare and utilize (scientific) data in decision-making, establishing key measures, and<br />
negotiating or solving trade barrier problems.<br />
Regulatory Framework and Existing Laws Related to <strong>Agricultural</strong> <strong>Biotechnology</strong>, <strong>Business</strong>,<br />
and Products (including GMOs)<br />
<strong>Agricultural</strong> biotechnology is relatively new in Thailand; the existing laws, mainly under<br />
the Ministry of Agriculture and Cooperatives, that are summarized in Table 1 have been implemented<br />
provisionally until new specific laws are issued. In addition, there are two guidelines:<br />
Genetic Engineering and <strong>Biotechnology</strong> <strong>for</strong> Laboratory Work and Genetic Engineering and<br />
<strong>Biotechnology</strong> <strong>for</strong> Field Work and Planned Release, approved in 1992 and implemented under<br />
the National Biosafety Committee (NBC) established in 1993. These guidelines were reviewed<br />
and published as Biosafety Guidelines <strong>for</strong> Work Related to Modern <strong>Biotechnology</strong> or Genetic<br />
Engineering in November 2004. Regulations <strong>for</strong> food containing ingredients derived from<br />
GMOs were drafted in 2002 by the Food and Drug Administration (FDA). They became a<br />
Ministerial Notification in 2002 and have been in <strong>for</strong>ce since May 11, 2003. Only GM soybean<br />
and corn are imported <strong>for</strong> food and feed production. The threshold level is 5% of DNA or<br />
protein from each of the top three ingredients, and each ingredient should be more than 5% by<br />
weight of product. The product should be labeled as Genetically Modified, <strong>for</strong> example, Gene-